Sunday Poster Session
Category: Stomach
P1676 - Atezolizumab-Induced Rapidly Progressive Gastritis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Vanessa F. Eller, MD
University of Arizona College of Medicine
Phoenix, AZ
Presenting Author(s)
Vanessa F. Eller, MD1, Elvis J. Arteaga, MD1, Ahmed Abidali, DO2, Bianca Arellano, MD3, Jen-Jung Pan, MD, PhD4
1University of Arizona College of Medicine, Phoenix, AZ; 2Abrazo Health, Glendale, AZ; 3Banner University Medical Center, Phoenix, AZ; 4Banner - University of Arizona, Phoenix, AZ
Introduction: Programmed cell death protein 1 (PD-1) and its associated ligand PD-L1 are commonly utilized regulatory proteins that are targeted in cancer immunotherapy. Despite its vast clinical benefits, these new therapies are not without their immune-related adverse effects. Gastrointestinal toxicities are not uncommon, with colitis being the most frequently reported and upper gastrointestinal effects having more limited documentation in the literature. In this case, we present a patient on a PD-L1 antagonist, atezolizumab, who initially presented with dysphagia and normal gross stomach pathology with rapidly progressive onset of his macroscopic gastritis.
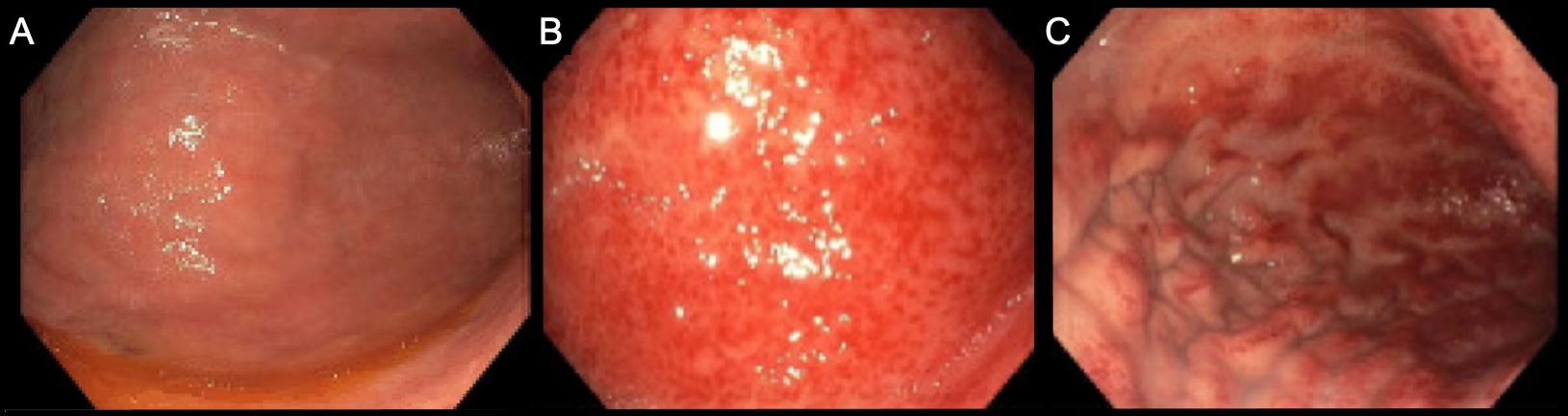
Case Description/Methods: The patient is a 54-year-old gentleman with a past medical history of hepatocellular carcinoma on atezolizumab therapy scheduled every 3 weeks, which he was tolerating well and mounting an appropriate response. He presented with one month of progressive dysphagia. An esophagogastroduodenoscopy (EGD) was performed and there were no endoluminal lesions noted in the esophagus or stomach (Figure A) to explain the patient's dysphagia. Repeat EGD 5 days later found diffuse and moderately erythematous mucosa in the cardia, the gastric fundus, and the gastric body (Figures B & C). The biopsy showed findings consistent with active gastritis with evidence of inflammation as well as scattered intraepithelial lymphocytes and neutrophils. The patient’s immunotherapy was discontinued due to possible immunotherapy-induced autoimmune disorder. The patient was considered too risky to rechallenge with immunotherapy and was discharged with instructions to follow up with oncology.
Discussion: In a literature review performed on 25 cases of immunotherapy-mediated gastritis, the average time to onset is about 29 weeks. This patient’s gastritis symptoms and mucosal findings had a temporal delay; however, the mucosal changes manifested within only 5 days since the last EGD and only 2 weeks since the previous infusion. While immunotherapy-mediated gastritis has limited reports in the literature, the increase in use of this novel therapy will likely alter the prevalence of disease. Timely diagnosis of this condition is essential given the risk of severe complications or even death. Thus, it is imperative to be aware of the adverse effects of immune-mediated gastritis when treating patients on immunotherapy. A biopsy should always be performed in those on immunotherapy, even if macroscopic changes are not noted on EGD.
Disclosures:
Vanessa F. Eller, MD1, Elvis J. Arteaga, MD1, Ahmed Abidali, DO2, Bianca Arellano, MD3, Jen-Jung Pan, MD, PhD4. P1676 - Atezolizumab-Induced Rapidly Progressive Gastritis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Arizona College of Medicine, Phoenix, AZ; 2Abrazo Health, Glendale, AZ; 3Banner University Medical Center, Phoenix, AZ; 4Banner - University of Arizona, Phoenix, AZ
Introduction: Programmed cell death protein 1 (PD-1) and its associated ligand PD-L1 are commonly utilized regulatory proteins that are targeted in cancer immunotherapy. Despite its vast clinical benefits, these new therapies are not without their immune-related adverse effects. Gastrointestinal toxicities are not uncommon, with colitis being the most frequently reported and upper gastrointestinal effects having more limited documentation in the literature. In this case, we present a patient on a PD-L1 antagonist, atezolizumab, who initially presented with dysphagia and normal gross stomach pathology with rapidly progressive onset of his macroscopic gastritis.
Case Description/Methods: The patient is a 54-year-old gentleman with a past medical history of hepatocellular carcinoma on atezolizumab therapy scheduled every 3 weeks, which he was tolerating well and mounting an appropriate response. He presented with one month of progressive dysphagia. An esophagogastroduodenoscopy (EGD) was performed and there were no endoluminal lesions noted in the esophagus or stomach (Figure A) to explain the patient's dysphagia. Repeat EGD 5 days later found diffuse and moderately erythematous mucosa in the cardia, the gastric fundus, and the gastric body (Figures B & C). The biopsy showed findings consistent with active gastritis with evidence of inflammation as well as scattered intraepithelial lymphocytes and neutrophils. The patient’s immunotherapy was discontinued due to possible immunotherapy-induced autoimmune disorder. The patient was considered too risky to rechallenge with immunotherapy and was discharged with instructions to follow up with oncology.
Discussion: In a literature review performed on 25 cases of immunotherapy-mediated gastritis, the average time to onset is about 29 weeks. This patient’s gastritis symptoms and mucosal findings had a temporal delay; however, the mucosal changes manifested within only 5 days since the last EGD and only 2 weeks since the previous infusion. While immunotherapy-mediated gastritis has limited reports in the literature, the increase in use of this novel therapy will likely alter the prevalence of disease. Timely diagnosis of this condition is essential given the risk of severe complications or even death. Thus, it is imperative to be aware of the adverse effects of immune-mediated gastritis when treating patients on immunotherapy. A biopsy should always be performed in those on immunotherapy, even if macroscopic changes are not noted on EGD.

Figure: FIGURE: Images obtained from upper endoscopy: (A) Gastric body mucosa (1 week and 2 days after last immunotherapy treatment), (B & C) Gastric body mucosa 5 days later (2 weeks after last immunotherapy treatment).
Disclosures:
Vanessa Eller indicated no relevant financial relationships.
Elvis Arteaga indicated no relevant financial relationships.
Ahmed Abidali indicated no relevant financial relationships.
Bianca Arellano indicated no relevant financial relationships.
Jen-Jung Pan indicated no relevant financial relationships.
Vanessa F. Eller, MD1, Elvis J. Arteaga, MD1, Ahmed Abidali, DO2, Bianca Arellano, MD3, Jen-Jung Pan, MD, PhD4. P1676 - Atezolizumab-Induced Rapidly Progressive Gastritis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
