Sunday Poster Session
Category: Stomach
P1604 - Response of Autoimmune Gastroparesis to Immunomodulation
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Madison Simons, PhD
Cleveland Clinic
Cleveland, OH
Presenting Author(s)
Award: Presidential Poster Award
Madison Simons, PhD1, Jack Loesch, BA1, Eyad Hamza, MS1, Anthony J. Lembo, MD2, Michael Cline, DO3, Matthew Allemang, MD1, Andrew D. Grubic, DO1
1Cleveland Clinic, Cleveland, OH; 2Digestive Disease Institute, Cleveland Clinic, Cleveland, OH; 3Cleveland Clinic Foundation, Cleveland, OH
Introduction: Gastroparesis is a chronic digestive condition in which a delay in gastric emptying is associated with symptoms including early satiety, excessive fullness, nausea, vomiting, and abdominal pain. Recent evidence suggests autoimmune factors may be involved in the development of gastroparesis, a subtype known as autoimmune gastrointestinal dysmotility (AGID). Additionally, small open label studies in gastroparetic patients with autoimmune markers have demonstrated intravenous immunoglobulin (IVIG) therapy leads to improvement in symptoms and gastric emptying. We aimed to evaluate the effects of IVIG therapy on symptom severity in patients with gastroparesis.
Methods: We conducted a retrospective case series involving patients with gastroparesis and seropositive autoimmune markers through medical chart review. All patients had evidence of delayed gastric emptying via gastric scintigraphy and had evidence of autoimmune dysfunction through seropositive antibody bloodwork, including glutamic acid decarboxylase (GAD), neuronal voltage-gated calcium channel, acetylcholine receptor, and neuronal voltage gated potassium channel autoantibodies. All patients received at least 12 weeks of IVIG therapy. Gastroparesis Cardinal Symptom Index (GCSI) scores were collected pre- and post-IVIG treatment. The study was approved by the Cleveland Clinic IRB.
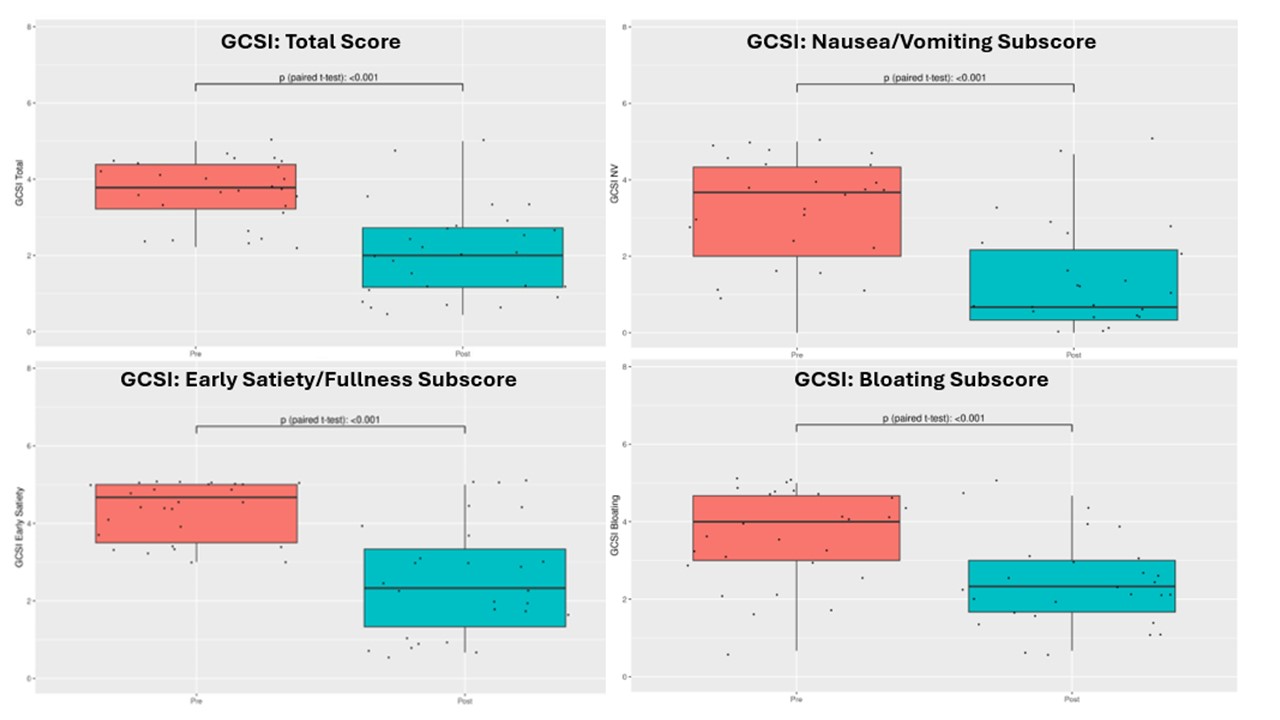
Results: We analyzed 27 patients with AGID. The total sample was 96.3% female; 81.5% White; mean age=39.0 (SD=13.4). 44.4% had a per-oral pyloromyotomy (POP) prior to IVIG therapy. GAD was the most common serum abnormality (44%). Gastric scintigraphy mean 4-hour retention was 38.6% (SD=24.4%). Wireless motility capsule gastric emptying time (GET) mean was 677.0 min (SD=585.5). Patients reported early satiety to be the most problematic symptom according to GCSI (M=4.3, SD=0.73). Following IVIG therapy, mean GCSI scores improved by over 1.5 points (pre-IVIG: 3.67, post-IVIG: 2.10, p<0.0001). 67% had an improvement of ≥1 on the GCSI following IVIG therapy.
Discussion: In this retrospective analysis of a small cohort of patients with AGID, IVIG therapy significantly improved symptoms. Randomized, placebo-controlled trials are needed to better understand the effectiveness of IVIG in treating AGID.
Disclosures:
Madison Simons, PhD1, Jack Loesch, BA1, Eyad Hamza, MS1, Anthony J. Lembo, MD2, Michael Cline, DO3, Matthew Allemang, MD1, Andrew D. Grubic, DO1. P1604 - Response of Autoimmune Gastroparesis to Immunomodulation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Madison Simons, PhD1, Jack Loesch, BA1, Eyad Hamza, MS1, Anthony J. Lembo, MD2, Michael Cline, DO3, Matthew Allemang, MD1, Andrew D. Grubic, DO1
1Cleveland Clinic, Cleveland, OH; 2Digestive Disease Institute, Cleveland Clinic, Cleveland, OH; 3Cleveland Clinic Foundation, Cleveland, OH
Introduction: Gastroparesis is a chronic digestive condition in which a delay in gastric emptying is associated with symptoms including early satiety, excessive fullness, nausea, vomiting, and abdominal pain. Recent evidence suggests autoimmune factors may be involved in the development of gastroparesis, a subtype known as autoimmune gastrointestinal dysmotility (AGID). Additionally, small open label studies in gastroparetic patients with autoimmune markers have demonstrated intravenous immunoglobulin (IVIG) therapy leads to improvement in symptoms and gastric emptying. We aimed to evaluate the effects of IVIG therapy on symptom severity in patients with gastroparesis.
Methods: We conducted a retrospective case series involving patients with gastroparesis and seropositive autoimmune markers through medical chart review. All patients had evidence of delayed gastric emptying via gastric scintigraphy and had evidence of autoimmune dysfunction through seropositive antibody bloodwork, including glutamic acid decarboxylase (GAD), neuronal voltage-gated calcium channel, acetylcholine receptor, and neuronal voltage gated potassium channel autoantibodies. All patients received at least 12 weeks of IVIG therapy. Gastroparesis Cardinal Symptom Index (GCSI) scores were collected pre- and post-IVIG treatment. The study was approved by the Cleveland Clinic IRB.
Results: We analyzed 27 patients with AGID. The total sample was 96.3% female; 81.5% White; mean age=39.0 (SD=13.4). 44.4% had a per-oral pyloromyotomy (POP) prior to IVIG therapy. GAD was the most common serum abnormality (44%). Gastric scintigraphy mean 4-hour retention was 38.6% (SD=24.4%). Wireless motility capsule gastric emptying time (GET) mean was 677.0 min (SD=585.5). Patients reported early satiety to be the most problematic symptom according to GCSI (M=4.3, SD=0.73). Following IVIG therapy, mean GCSI scores improved by over 1.5 points (pre-IVIG: 3.67, post-IVIG: 2.10, p<0.0001). 67% had an improvement of ≥1 on the GCSI following IVIG therapy.
Discussion: In this retrospective analysis of a small cohort of patients with AGID, IVIG therapy significantly improved symptoms. Randomized, placebo-controlled trials are needed to better understand the effectiveness of IVIG in treating AGID.

Figure: Figure 1. Comparison of pre- versus post-IVIG therapy GCSI score and subscales
Disclosures:
Madison Simons indicated no relevant financial relationships.
Jack Loesch: Eli Lilly and Company – Stock-publicly held company(excluding mutual/index funds).
Eyad Hamza indicated no relevant financial relationships.
Anthony Lembo: Allurion – Stock Options. Bristol Myers Squibb – Stock Options. Johnson & Johnson – Stock Options. Vibrant Advisory Board – Advisory Committee/Board Member.
Michael Cline indicated no relevant financial relationships.
Matthew Allemang indicated no relevant financial relationships.
Andrew Grubic indicated no relevant financial relationships.
Madison Simons, PhD1, Jack Loesch, BA1, Eyad Hamza, MS1, Anthony J. Lembo, MD2, Michael Cline, DO3, Matthew Allemang, MD1, Andrew D. Grubic, DO1. P1604 - Response of Autoimmune Gastroparesis to Immunomodulation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

