Sunday Poster Session
Category: Liver
P1170 - Racial Disparity in Hepatitis C Clinical Trials in the United States: Trend Over Two-Decades
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Ayusha Poudel, MD
Cook County Health
Chicago, IL
Presenting Author(s)
Ayusha Poudel, MD1, Anurag Adhikari, MD2, Sajana Poudel, MD3
1Cook County Health, Chicago, IL; 2NYC Health + Hospitals/Jacobi, New York, NY; 3John H. Stroger, Jr. Hospital of Cook County, Chicago, IL
Introduction: Clinical trials are undertaken to determine the suitability of a clinical therapy to a medical condition. It is imperative that the participants in the clinical trials reflect the general population so that the results can be generalized. It is especially important in disease conditions like Hepatitis B and C which disproportionately affects the marginalized racial groups.
Methods: We searched the clinical trials listed in clinicaltrial.gov for Phase 3 and Phase 4 clinical trials related to Hepatitis C. The inclusion criteria included trials performed exclusively in the United States. We collected data regarding the gender and racial distribution of each of the clinical trials. Linear and logistic regression analyses were done using JASP.
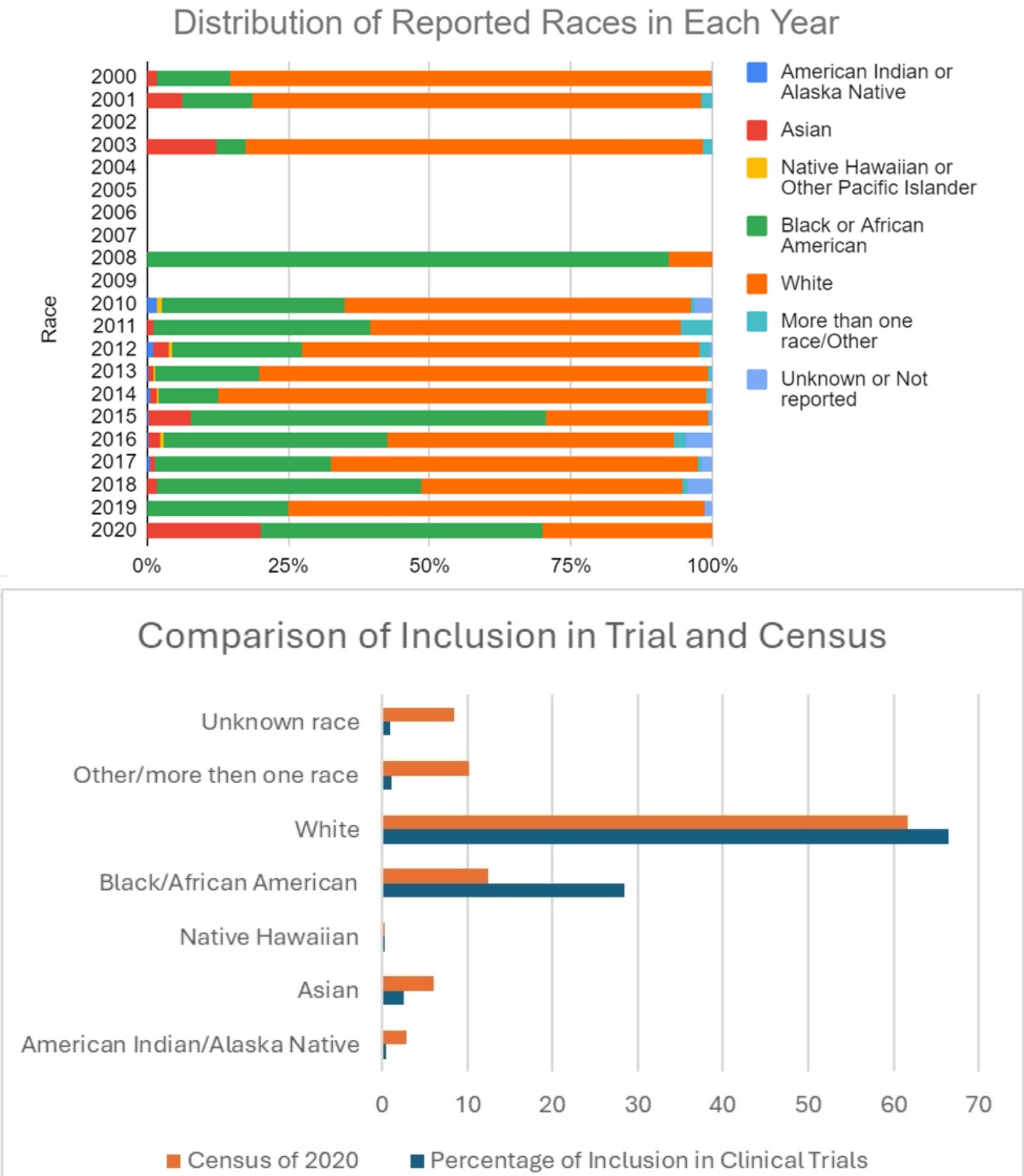
Results: A total of 155 studies were conducted for Hepatitis C, out of which 81 studies fulfilled the inclusion criteria. Gender was reported in 98.77% (80/81) studies and racial profile was reported in only 66.67% (54/81) studies. Among the studies reporting gender 67.10% (7877/11740) were males and 32.90% (3863/7877) were females. There were a total of 0.42% (28/6646) American Indian/Alaska Native, 2.53% (168/6646) Asian, 0.24% (16/6646) Native Hawaiian, 28.39% (1887/6646) Black/African American, 66.46% (4417/6646) White, 1.11% (74/6646) Other/more than one race and 0.84% (56/6646) Unknown race reported in the studies that reported race. Linear regression model demonstrated that the inclusion of Only Black or African American was increasing with OR [89.857, 95% CI 23.57-156.15] from the year 2000 to 2020. None of the other race categories showed a statistically significant rise in inclusivity in the clinical trials. Logistic regression revealed that there was a statistically significant increase in inclusion of various races with progression of year, OR [1.27, 95% CI 1.13-1.43].
Discussion: The highest rate of Hepatitis C cases reported in United States is among the American Indian/Alaska Native race with 2.1 cases per 100,000 population. However, in the clinical trials they are represented at a dismal rate of 0.42% just above Native Hawaiian at 0.24%. There is a substantial disparity among representation of native population in clinical trials in the United States even among diseases of concern to them. Special focus should be made to improve the representation.
Disclosures:
Ayusha Poudel, MD1, Anurag Adhikari, MD2, Sajana Poudel, MD3. P1170 - Racial Disparity in Hepatitis C Clinical Trials in the United States: Trend Over Two-Decades, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Cook County Health, Chicago, IL; 2NYC Health + Hospitals/Jacobi, New York, NY; 3John H. Stroger, Jr. Hospital of Cook County, Chicago, IL
Introduction: Clinical trials are undertaken to determine the suitability of a clinical therapy to a medical condition. It is imperative that the participants in the clinical trials reflect the general population so that the results can be generalized. It is especially important in disease conditions like Hepatitis B and C which disproportionately affects the marginalized racial groups.
Methods: We searched the clinical trials listed in clinicaltrial.gov for Phase 3 and Phase 4 clinical trials related to Hepatitis C. The inclusion criteria included trials performed exclusively in the United States. We collected data regarding the gender and racial distribution of each of the clinical trials. Linear and logistic regression analyses were done using JASP.
Results: A total of 155 studies were conducted for Hepatitis C, out of which 81 studies fulfilled the inclusion criteria. Gender was reported in 98.77% (80/81) studies and racial profile was reported in only 66.67% (54/81) studies. Among the studies reporting gender 67.10% (7877/11740) were males and 32.90% (3863/7877) were females. There were a total of 0.42% (28/6646) American Indian/Alaska Native, 2.53% (168/6646) Asian, 0.24% (16/6646) Native Hawaiian, 28.39% (1887/6646) Black/African American, 66.46% (4417/6646) White, 1.11% (74/6646) Other/more than one race and 0.84% (56/6646) Unknown race reported in the studies that reported race. Linear regression model demonstrated that the inclusion of Only Black or African American was increasing with OR [89.857, 95% CI 23.57-156.15] from the year 2000 to 2020. None of the other race categories showed a statistically significant rise in inclusivity in the clinical trials. Logistic regression revealed that there was a statistically significant increase in inclusion of various races with progression of year, OR [1.27, 95% CI 1.13-1.43].
Discussion: The highest rate of Hepatitis C cases reported in United States is among the American Indian/Alaska Native race with 2.1 cases per 100,000 population. However, in the clinical trials they are represented at a dismal rate of 0.42% just above Native Hawaiian at 0.24%. There is a substantial disparity among representation of native population in clinical trials in the United States even among diseases of concern to them. Special focus should be made to improve the representation.

Figure: Racial Distribution with Year and Census Data
Disclosures:
Ayusha Poudel indicated no relevant financial relationships.
Anurag Adhikari indicated no relevant financial relationships.
Sajana Poudel indicated no relevant financial relationships.
Ayusha Poudel, MD1, Anurag Adhikari, MD2, Sajana Poudel, MD3. P1170 - Racial Disparity in Hepatitis C Clinical Trials in the United States: Trend Over Two-Decades, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
