Sunday Poster Session
Category: IBD
P0956 - Analyzing the Racial Diversity of Clinical Trials in IBD: A Systematic Review
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Alexander Garcia, DO
Cooper University Hospital
Camden, NJ
Presenting Author(s)
Alexander Garcia, DO1, Avneet Singh, DO1, Queenzy Jover, BSN, RN2, Zehara Abidi, BS, MS3, Aleeza Shakeel, BS4, Anand Thakkar, BS5, Krystal Hunter, PhD, MBA6, Stephanie Rivera Morales, MD7, Krysta Contino, MD8
1Cooper University Hospital, Camden, NJ; 2Virtua Health System, Camden, NJ; 3Touro College of Osteopathic Medicine, New York, NY; 4Cooper Medical School of Rowan University, Camden, NJ; 5Philadelphia College of Osteopathic Medicine, Philadelphia, PA; 6Cooper University Health Care, Camden, NJ; 7Lankenau Medical Center, Wynnewood, PA; 8Digestive Health Institute at Cooper University Hospital, Mount Laurel, NJ
Introduction: North America leads the world in inflammatory bowel disease (IBD) rates with about 2.4 million people in the United States (US) affected. The burden of IBD will likely rise given increasing life expectancy. At present, there are few studies characterizing the racial and ethnic representation in IBD clinical trials. Those that exist suggest racial disparities in minority enrollment. Our study aim is to evaluate the racial makeup of US-based clinical trials in IBD to determine if enrollment disparities persist.
Methods: A systematic review was conducted on all available adult IBD trials on Clinicaltrials.gov from January 2010 to January 2024. Studies were excluded if they were not yet complete, lacked reporting of race and ethnicity, were completed outside of the US, or used non-drug therapy as an intervention. Data extraction and analysis were independently performed by two reviewers. Data about age, gender, race, and ethnicity were recorded for included trials. The racial and ethnic burden of IBD in the United States was calculated using prevalence rates and the 2020 US Census.
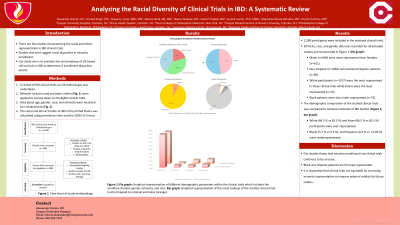
Results: A total of 198 clinical trials were retrieved and 20 met inclusion criteria. These 20 studies included a total of 1,280 participants. Of the 20 trials, eight were on ulcerative colitis (UC), eight on Crohn’s disease (CD), and four were on both UC and CD. Male (n= 669; 52.3 %) representation was greater compared to females (n= 611; 47.7 %). Ethnicity data was available in 17/20 of the clinical trials with Non-Hispanic (n= 1082; 91.7 %) participants outnumbering Hispanics (n= 98; 8.3 %). Racial composition mimicked ethnic disparities with the highest representation among White participants and lowest among Asians (N = 1280: White= 1107; 86.5 %, Black=73; 5.7 %, Asian= 72; 5.6 %, Other=28; 2.2 %). When comparing the demographic composition of clinical trials to national estimates, Whites (86.5 % vs 83.3 %) and Asians (5.6 % vs. 3.27 %) were over-represented. Blacks (5.7 % vs 9.3 %), and Hispanics (8.3 % vs. 11.83 %) were underrepresented.
Discussion: Our systematic review has several key highlights. Of the studies that were excluded, 23 % did not report race and ethnicity. Additionally, we noted an overall low representation of Black and Hispanic participants in our included clinical trials. It is imperative that clinical trials are equitable and increase minority representation to increase the external validity of future studies.
Disclosures:
Alexander Garcia, DO1, Avneet Singh, DO1, Queenzy Jover, BSN, RN2, Zehara Abidi, BS, MS3, Aleeza Shakeel, BS4, Anand Thakkar, BS5, Krystal Hunter, PhD, MBA6, Stephanie Rivera Morales, MD7, Krysta Contino, MD8. P0956 - Analyzing the Racial Diversity of Clinical Trials in IBD: A Systematic Review, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Cooper University Hospital, Camden, NJ; 2Virtua Health System, Camden, NJ; 3Touro College of Osteopathic Medicine, New York, NY; 4Cooper Medical School of Rowan University, Camden, NJ; 5Philadelphia College of Osteopathic Medicine, Philadelphia, PA; 6Cooper University Health Care, Camden, NJ; 7Lankenau Medical Center, Wynnewood, PA; 8Digestive Health Institute at Cooper University Hospital, Mount Laurel, NJ
Introduction: North America leads the world in inflammatory bowel disease (IBD) rates with about 2.4 million people in the United States (US) affected. The burden of IBD will likely rise given increasing life expectancy. At present, there are few studies characterizing the racial and ethnic representation in IBD clinical trials. Those that exist suggest racial disparities in minority enrollment. Our study aim is to evaluate the racial makeup of US-based clinical trials in IBD to determine if enrollment disparities persist.
Methods: A systematic review was conducted on all available adult IBD trials on Clinicaltrials.gov from January 2010 to January 2024. Studies were excluded if they were not yet complete, lacked reporting of race and ethnicity, were completed outside of the US, or used non-drug therapy as an intervention. Data extraction and analysis were independently performed by two reviewers. Data about age, gender, race, and ethnicity were recorded for included trials. The racial and ethnic burden of IBD in the United States was calculated using prevalence rates and the 2020 US Census.
Results: A total of 198 clinical trials were retrieved and 20 met inclusion criteria. These 20 studies included a total of 1,280 participants. Of the 20 trials, eight were on ulcerative colitis (UC), eight on Crohn’s disease (CD), and four were on both UC and CD. Male (n= 669; 52.3 %) representation was greater compared to females (n= 611; 47.7 %). Ethnicity data was available in 17/20 of the clinical trials with Non-Hispanic (n= 1082; 91.7 %) participants outnumbering Hispanics (n= 98; 8.3 %). Racial composition mimicked ethnic disparities with the highest representation among White participants and lowest among Asians (N = 1280: White= 1107; 86.5 %, Black=73; 5.7 %, Asian= 72; 5.6 %, Other=28; 2.2 %). When comparing the demographic composition of clinical trials to national estimates, Whites (86.5 % vs 83.3 %) and Asians (5.6 % vs. 3.27 %) were over-represented. Blacks (5.7 % vs 9.3 %), and Hispanics (8.3 % vs. 11.83 %) were underrepresented.
Discussion: Our systematic review has several key highlights. Of the studies that were excluded, 23 % did not report race and ethnicity. Additionally, we noted an overall low representation of Black and Hispanic participants in our included clinical trials. It is imperative that clinical trials are equitable and increase minority representation to increase the external validity of future studies.
Disclosures:
Alexander Garcia indicated no relevant financial relationships.
Avneet Singh indicated no relevant financial relationships.
Queenzy Jover indicated no relevant financial relationships.
Zehara Abidi indicated no relevant financial relationships.
Aleeza Shakeel indicated no relevant financial relationships.
Anand Thakkar indicated no relevant financial relationships.
Krystal Hunter indicated no relevant financial relationships.
Stephanie Rivera Morales indicated no relevant financial relationships.
Krysta Contino indicated no relevant financial relationships.
Alexander Garcia, DO1, Avneet Singh, DO1, Queenzy Jover, BSN, RN2, Zehara Abidi, BS, MS3, Aleeza Shakeel, BS4, Anand Thakkar, BS5, Krystal Hunter, PhD, MBA6, Stephanie Rivera Morales, MD7, Krysta Contino, MD8. P0956 - Analyzing the Racial Diversity of Clinical Trials in IBD: A Systematic Review, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
