Sunday Poster Session
Category: IBD
P0961 - Exploring Dual-Targeted Therapy in the Management of Moderate to Severe Inflammatory Bowel Disease: A Retrospective Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- SB
Sonya Bhaskar, MD
University of South Florida
Tampa, FL
Presenting Author(s)
Sonya Bhaskar, MD1, Zachary Makovich, MD2, Emily Coughlin, MPH1, Rahul Mhaskar, MPH, PhD1, Jennifer L. Seminerio, MD3
1University of South Florida, Tampa, FL; 2University of South Florida Morsani College of Medicine, Tampa, FL; 3USF Health Morsani, Tampa, FL
Introduction: Inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn's disease (CD), often leads to significant morbidity in patients with moderate to severe disease. Despite the efficacy of biologics and small molecules in achieving remission, a substantial subset of patients have refractory disease or extra-intestinal manifestations. Combining therapies with different mechanisms of action could provide an additive or synergistic benefit, potentially overcoming treatment resistance. This study evaluates the safety and efficacy of dual-targeted therapy in a cohort of IBD patients at the USF Health Inflammatory Bowel Disease Center.
Methods: This retrospective cohort study analyzed 79 patients with UC or CD treated with dual-targeted therapy at the University of South Florida from October 2018 to August 2023. Data collected included demographic information, disease characteristics, previous treatments, and clinical outcomes. Primary outcomes focused on endoscopic, radiographic, and patient-reported clinical improvements, while secondary outcomes assessed safety profiles.
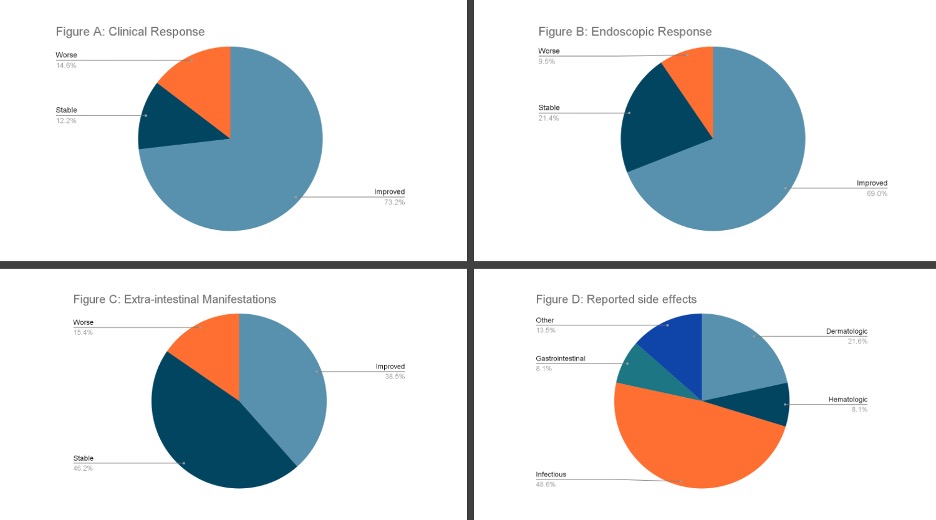
Results: Among the 79 patients (42 UC, 37 CD), 97 dual-targeted therapy cases were identified, primarily combining a biologic with a JAK inhibitor (90.7%). The median duration of therapy was 39.1 weeks. Endoscopic improvement was noted in 69% of cases. Clinical improvement was reported by 73.2% of patients, and significant reductions in ESR, CRP, and albumin levels were observed. Non-obese patients demonstrated better outcomes than their obese counterparts. Adverse events occurred in 37 cases, with the most common being upper respiratory tract infections and dermatologic issues. No thromboembolic events were reported.
Discussion: Dual-targeted therapy showed efficacy in improving clinical and endoscopic outcomes in severe, refractory IBD patients, with an acceptable safety profile. The study supports the potential of this treatment approach, though further research is needed to confirm these findings and explore optimal combinations. Limitations include the retrospective nature and small sample size, highlighting the need for larger, controlled studies.
Disclosures:
Sonya Bhaskar, MD1, Zachary Makovich, MD2, Emily Coughlin, MPH1, Rahul Mhaskar, MPH, PhD1, Jennifer L. Seminerio, MD3. P0961 - Exploring Dual-Targeted Therapy in the Management of Moderate to Severe Inflammatory Bowel Disease: A Retrospective Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of South Florida, Tampa, FL; 2University of South Florida Morsani College of Medicine, Tampa, FL; 3USF Health Morsani, Tampa, FL
Introduction: Inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn's disease (CD), often leads to significant morbidity in patients with moderate to severe disease. Despite the efficacy of biologics and small molecules in achieving remission, a substantial subset of patients have refractory disease or extra-intestinal manifestations. Combining therapies with different mechanisms of action could provide an additive or synergistic benefit, potentially overcoming treatment resistance. This study evaluates the safety and efficacy of dual-targeted therapy in a cohort of IBD patients at the USF Health Inflammatory Bowel Disease Center.
Methods: This retrospective cohort study analyzed 79 patients with UC or CD treated with dual-targeted therapy at the University of South Florida from October 2018 to August 2023. Data collected included demographic information, disease characteristics, previous treatments, and clinical outcomes. Primary outcomes focused on endoscopic, radiographic, and patient-reported clinical improvements, while secondary outcomes assessed safety profiles.
Results: Among the 79 patients (42 UC, 37 CD), 97 dual-targeted therapy cases were identified, primarily combining a biologic with a JAK inhibitor (90.7%). The median duration of therapy was 39.1 weeks. Endoscopic improvement was noted in 69% of cases. Clinical improvement was reported by 73.2% of patients, and significant reductions in ESR, CRP, and albumin levels were observed. Non-obese patients demonstrated better outcomes than their obese counterparts. Adverse events occurred in 37 cases, with the most common being upper respiratory tract infections and dermatologic issues. No thromboembolic events were reported.
Discussion: Dual-targeted therapy showed efficacy in improving clinical and endoscopic outcomes in severe, refractory IBD patients, with an acceptable safety profile. The study supports the potential of this treatment approach, though further research is needed to confirm these findings and explore optimal combinations. Limitations include the retrospective nature and small sample size, highlighting the need for larger, controlled studies.

Figure: Figure A: Reported clinical responses in patients with inflammatory bowel disease at USF after receiving dual targeted therapy
Figure B: Endoscopic response in patients with inflammatory bowel disease at USF after receiving dual targeted therapy
Figure C: Effects on extra-intestinal manifestations in patients with inflammatory bowel disease at USF after receiving dual targeted therapy
Figure D: Reported side effects in patients with inflammatory bowel disease at USF after receiving dual targeted therapy
Figure B: Endoscopic response in patients with inflammatory bowel disease at USF after receiving dual targeted therapy
Figure C: Effects on extra-intestinal manifestations in patients with inflammatory bowel disease at USF after receiving dual targeted therapy
Figure D: Reported side effects in patients with inflammatory bowel disease at USF after receiving dual targeted therapy
Disclosures:
Sonya Bhaskar indicated no relevant financial relationships.
Zachary Makovich indicated no relevant financial relationships.
Emily Coughlin indicated no relevant financial relationships.
Rahul Mhaskar indicated no relevant financial relationships.
Jennifer Seminerio: Abbvie – Advisor or Review Panel Member, Consultant, Speakers Bureau. Bristol Meyers Squibb – Advisor or Review Panel Member, Consultant, Speakers Bureau. Johnson and Johnson (Janssen) – Advisor or Review Panel Member, Consultant, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Consultant, Speakers Bureau. Takeda – Advisor or Review Panel Member, Consultant, Speakers Bureau.
Sonya Bhaskar, MD1, Zachary Makovich, MD2, Emily Coughlin, MPH1, Rahul Mhaskar, MPH, PhD1, Jennifer L. Seminerio, MD3. P0961 - Exploring Dual-Targeted Therapy in the Management of Moderate to Severe Inflammatory Bowel Disease: A Retrospective Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
