Sunday Poster Session
Category: IBD
P0875 - Comparative Outcomes of Eosinophilic Esophagitis in Patients With and Without Inflammatory Bowel Disease: A Propensity-Matched Cohort Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Himsikhar Khataniar, MD
Allegheny General Hospital
Pittsburgh, PA
Presenting Author(s)
Himsikhar Khataniar, MD1, Aakash Desai, MD2, Jana G. Hashash, MD, MSc3, Fjona Tabaku, MD1, Francis A. Farraye, MD, MSc3, Gursimran S. Kochhar, MD1
1Allegheny General Hospital, Pittsburgh, PA; 2Mayo Clinic, Pittsburgh, PA; 3Mayo Clinic, Jacksonville, FL
Introduction: Eosinophilic esophagitis (EoE) and inflammatory bowel disease (IBD) are recognized as immunologically mediated conditions affecting the gastrointestinal tract. Previous investigations have yielded inconsistent results regarding the risk of EoE in individuals with IBD, and the reverse association has been infrequently explored. Despite these findings, the influence of concurrent IBD on the progression of EoE remains undocumented.
Methods: This study employed a retrospective cohort design, utilizing the TriNetX database to examine the 5-year outcomes in patients with coexisting EoE and IBD (EoE-IBD cohort), compared to those diagnosed with EoE without IBD (Control cohort) between 2013 and 2022. The primary objective was to assess the risk of a composite outcome which included esophageal stricture requiring dilation and/or use of dupilumab within 5 years. The secondary objective was to assess frequency of esophageal dilations and risk of esophageal food impactions between the two cohorts and influence of IBD medications on the primary outcome. 1:1 propensity score matching was performed for age, gender, race and EoE medications (PPI and topical steroids). Risk was presented as the adjusted odds ratio (aOR) with 95% confidence intervals (CI).
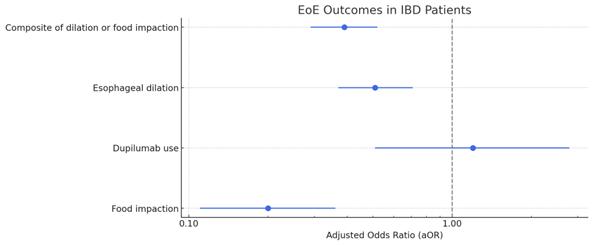
Results: The study included a total number of 887 (mean age 34 +/- 18.3, female sex 38.1%, White race 81.8%) patients diagnosed with both EoE-IBD cohort. Following propensity score matching, there was lower risk of a composite outcome of esophageal dilation and/or Dupilumab use (aOR: 0.39, CI: 0.29-0.52) between the two cohorts. There was no difference in the risk of esophageal dilations alone (aOR: 0.51, CI: 0.37-0.71) and use of Dupilumab (aOR: 0.2, CI: 0.11-0.36) between the two cohorts. No significant differences were observed in the risk of food impactions (aOR: 0.2, CI: 0.11-0.36) between the two cohorts. Additionally, there was no difference in the mean number of dilations (p=0.24) required in both cohorts.
Discussion: Our findings suggest more favorable outcomes in patients with EoE and IBD compared to those with EoE alone. This may be related to immune-modifying therapies used for IBD, early diagnosis and close monitoring in patients with IBD and EoE.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Himsikhar Khataniar, MD1, Aakash Desai, MD2, Jana G. Hashash, MD, MSc3, Fjona Tabaku, MD1, Francis A. Farraye, MD, MSc3, Gursimran S. Kochhar, MD1. P0875 - Comparative Outcomes of Eosinophilic Esophagitis in Patients With and Without Inflammatory Bowel Disease: A Propensity-Matched Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Allegheny General Hospital, Pittsburgh, PA; 2Mayo Clinic, Pittsburgh, PA; 3Mayo Clinic, Jacksonville, FL
Introduction: Eosinophilic esophagitis (EoE) and inflammatory bowel disease (IBD) are recognized as immunologically mediated conditions affecting the gastrointestinal tract. Previous investigations have yielded inconsistent results regarding the risk of EoE in individuals with IBD, and the reverse association has been infrequently explored. Despite these findings, the influence of concurrent IBD on the progression of EoE remains undocumented.
Methods: This study employed a retrospective cohort design, utilizing the TriNetX database to examine the 5-year outcomes in patients with coexisting EoE and IBD (EoE-IBD cohort), compared to those diagnosed with EoE without IBD (Control cohort) between 2013 and 2022. The primary objective was to assess the risk of a composite outcome which included esophageal stricture requiring dilation and/or use of dupilumab within 5 years. The secondary objective was to assess frequency of esophageal dilations and risk of esophageal food impactions between the two cohorts and influence of IBD medications on the primary outcome. 1:1 propensity score matching was performed for age, gender, race and EoE medications (PPI and topical steroids). Risk was presented as the adjusted odds ratio (aOR) with 95% confidence intervals (CI).
Results: The study included a total number of 887 (mean age 34 +/- 18.3, female sex 38.1%, White race 81.8%) patients diagnosed with both EoE-IBD cohort. Following propensity score matching, there was lower risk of a composite outcome of esophageal dilation and/or Dupilumab use (aOR: 0.39, CI: 0.29-0.52) between the two cohorts. There was no difference in the risk of esophageal dilations alone (aOR: 0.51, CI: 0.37-0.71) and use of Dupilumab (aOR: 0.2, CI: 0.11-0.36) between the two cohorts. No significant differences were observed in the risk of food impactions (aOR: 0.2, CI: 0.11-0.36) between the two cohorts. Additionally, there was no difference in the mean number of dilations (p=0.24) required in both cohorts.
Discussion: Our findings suggest more favorable outcomes in patients with EoE and IBD compared to those with EoE alone. This may be related to immune-modifying therapies used for IBD, early diagnosis and close monitoring in patients with IBD and EoE.

Figure: Forest Plot depicting EoE outcomes in IBD patients
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Himsikhar Khataniar indicated no relevant financial relationships.
Aakash Desai indicated no relevant financial relationships.
Jana Hashash: Bristol Myers Squibb – Consultant.
Fjona Tabaku indicated no relevant financial relationships.
Francis Farraye: AbbVie – Consultant. Avalo Therapeutics – Consultant. Bausch – Advisor or Review Panel Member. BMS – Consultant. Braintree Labs – Consultant. DSMB for Lilly. – Sits on. Fresenius Kabi – Consultant. GI Reviewers and IBD Educational Group – independent contractor. GSK, Iterative Health, Janssen, Pfizer, Pharmacosmos, Sandoz Immunology, Sebela and Viatris – Consultant.
Gursimran Kochhar: Boston Scientific Endoscopy – Consultant. DigbI – Stock Options. Eli Lilli – Advisory Committee/Board Member, Speakers Bureau. Olympus Endoscopy – Consultant. Pentax Endoscopy – Consultant. Takeda – Consultant.
Himsikhar Khataniar, MD1, Aakash Desai, MD2, Jana G. Hashash, MD, MSc3, Fjona Tabaku, MD1, Francis A. Farraye, MD, MSc3, Gursimran S. Kochhar, MD1. P0875 - Comparative Outcomes of Eosinophilic Esophagitis in Patients With and Without Inflammatory Bowel Disease: A Propensity-Matched Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
