Sunday Poster Session
Category: Colorectal Cancer Prevention
P0450 - Hidden Hazards: Desiccant Canister Ingestion During Sodium Sulfate Bowel Preparation – A Case Report and Safety Recommendations
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

David Okuampa, MD
Louisiana State University Health
Shreveport, LA
Presenting Author(s)
Award: Presidential Poster Award
David Okuampa, MD1, Michelle Neice, MD2, Lena Kawji, MD2, James D. Morris, MD, FACG2
1Louisiana State University Health, Shreveport, LA; 2LSU Health, Shreveport, LA
Introduction: Desiccants, found in packets or cylinders, are used in medication containers to keep contents dry. While foreign body ingestion of these materials is common in children, it is increasingly reported in adults with significant complications. We present a case of a 46-year-old woman with an incidental finding of a desiccant canister in the cecum during a screening colonoscopy.
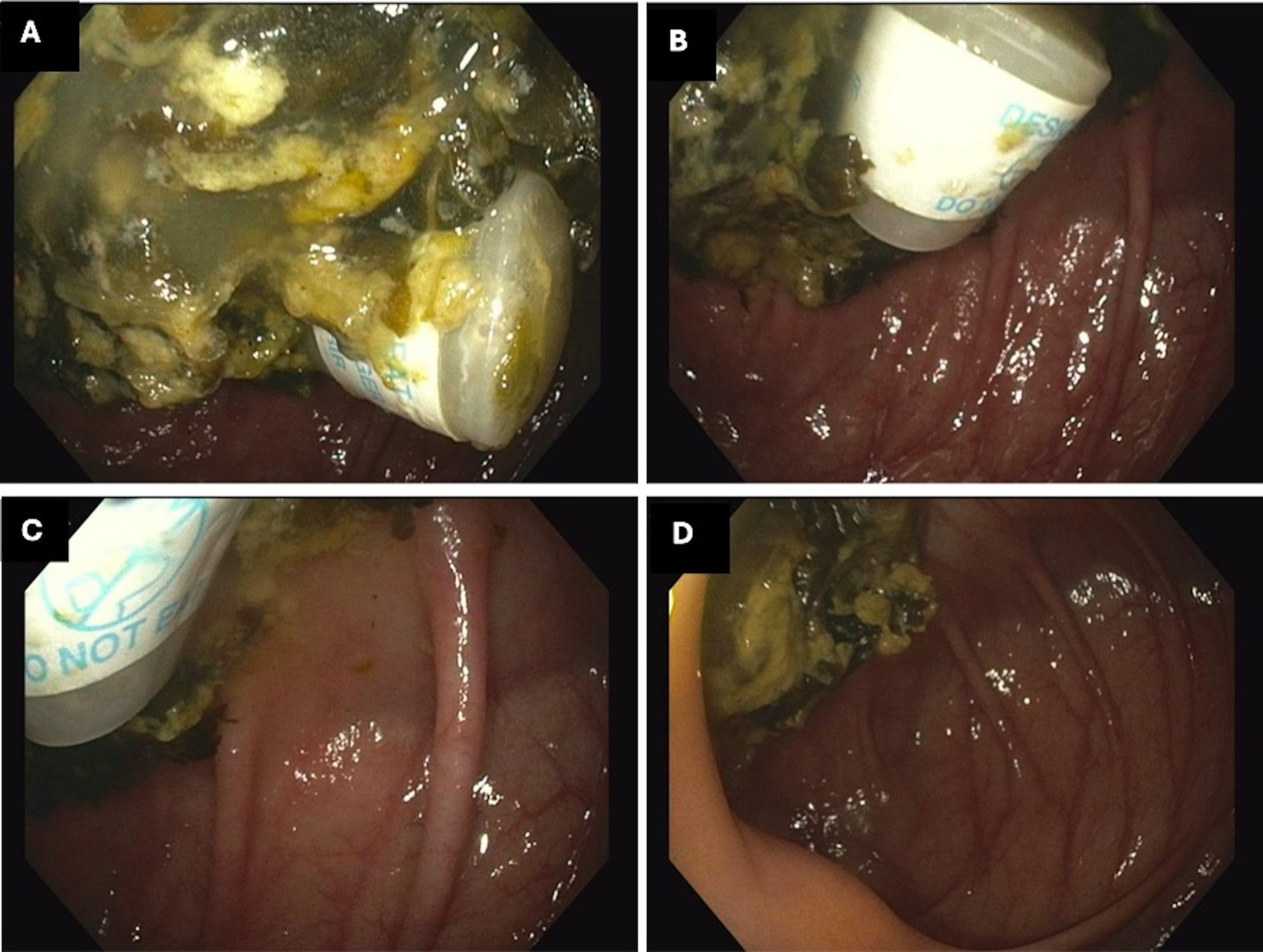
Case Description/Methods: A 46-year-old woman with chronic GERD underwent upper endoscopy for persistent epigastric pain and a routine screening colonoscopy. She used sodium sulfate, magnesium sulfate, and potassium chloride (sodium sulfate) tablets for bowel preparation. The colonoscopy revealed a single cylindrical desiccant canister labeled “DO NOT EAT” in the cecum, likely from one of the containers of bowel preparation tablets, which was retrieved with a cold snare (Figure 1a-d). She did not recall ingesting the canister and does not have any vision issues.
Discussion: Two cases of esophageal obstruction and one case of colonic obstruction involving cylindrical desiccant canisters have been reported in patients with preexisting gastrointestinal strictures. Our patient fortunately avoided this outcome, as her canister was successfully retrieved without complications. To our knowledge, this is the first reported case of desiccant canister ingestion involving a sodium sulfate tablet bowel preparation.
Sodium sulfate tablets, used for split-dose bowel prep before colonoscopy, come in two bottles, each containing 12 tablets and a cylindrical desiccant canister. Our patient took no other medication during her prep, indicating that the canister found in her cecum came from one of these bottles. Multiple factors, including the similar size and color of the canister and tablets, the need to take 12 tablets at a time, poor segregation of tablets and canister, and inadequate patient education about proper tablet administration, all could have contributed to this incident.
As the use of sodium sulfate tablets rises, so does the risk of desiccant canister ingestion, posing a greater danger to patients with esophageal or colonic strictures. This could deter patients from undergoing screening colonoscopies.
To enhance patient safety, manufacturers should anchor desiccant canisters to the bottle or lid, design them with distinct colors, labels, and shapes to differentiate them from tablets. Also, reducing the number of pills and bottles required for the regimen and improving patient education can significantly decrease these incidents.
Disclosures:
David Okuampa, MD1, Michelle Neice, MD2, Lena Kawji, MD2, James D. Morris, MD, FACG2. P0450 - Hidden Hazards: Desiccant Canister Ingestion During Sodium Sulfate Bowel Preparation – A Case Report and Safety Recommendations, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
David Okuampa, MD1, Michelle Neice, MD2, Lena Kawji, MD2, James D. Morris, MD, FACG2
1Louisiana State University Health, Shreveport, LA; 2LSU Health, Shreveport, LA
Introduction: Desiccants, found in packets or cylinders, are used in medication containers to keep contents dry. While foreign body ingestion of these materials is common in children, it is increasingly reported in adults with significant complications. We present a case of a 46-year-old woman with an incidental finding of a desiccant canister in the cecum during a screening colonoscopy.
Case Description/Methods: A 46-year-old woman with chronic GERD underwent upper endoscopy for persistent epigastric pain and a routine screening colonoscopy. She used sodium sulfate, magnesium sulfate, and potassium chloride (sodium sulfate) tablets for bowel preparation. The colonoscopy revealed a single cylindrical desiccant canister labeled “DO NOT EAT” in the cecum, likely from one of the containers of bowel preparation tablets, which was retrieved with a cold snare (Figure 1a-d). She did not recall ingesting the canister and does not have any vision issues.
Discussion: Two cases of esophageal obstruction and one case of colonic obstruction involving cylindrical desiccant canisters have been reported in patients with preexisting gastrointestinal strictures. Our patient fortunately avoided this outcome, as her canister was successfully retrieved without complications. To our knowledge, this is the first reported case of desiccant canister ingestion involving a sodium sulfate tablet bowel preparation.
Sodium sulfate tablets, used for split-dose bowel prep before colonoscopy, come in two bottles, each containing 12 tablets and a cylindrical desiccant canister. Our patient took no other medication during her prep, indicating that the canister found in her cecum came from one of these bottles. Multiple factors, including the similar size and color of the canister and tablets, the need to take 12 tablets at a time, poor segregation of tablets and canister, and inadequate patient education about proper tablet administration, all could have contributed to this incident.
As the use of sodium sulfate tablets rises, so does the risk of desiccant canister ingestion, posing a greater danger to patients with esophageal or colonic strictures. This could deter patients from undergoing screening colonoscopies.
To enhance patient safety, manufacturers should anchor desiccant canisters to the bottle or lid, design them with distinct colors, labels, and shapes to differentiate them from tablets. Also, reducing the number of pills and bottles required for the regimen and improving patient education can significantly decrease these incidents.

Figure: Figure 1A-C Single Desiccant Canister and undigested food in the Cecum with the "DO NOT EAT" label. Figure 1D Canister retrieved with cold snare (not pictured) with no sign of mucosal damage.
Disclosures:
David Okuampa indicated no relevant financial relationships.
Michelle Neice indicated no relevant financial relationships.
Lena Kawji indicated no relevant financial relationships.
James Morris indicated no relevant financial relationships.
David Okuampa, MD1, Michelle Neice, MD2, Lena Kawji, MD2, James D. Morris, MD, FACG2. P0450 - Hidden Hazards: Desiccant Canister Ingestion During Sodium Sulfate Bowel Preparation – A Case Report and Safety Recommendations, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

