Tuesday Poster Session
Category: Functional Bowel Disease
P4042 - Dose-Related Effects of Ricinoleic Acid (Castor Oil) on Gut Permeability in Healthy Participants: Provocative Test for Treatments Aimed at Restoring Barrier Function
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

David Y. Yang, MD
University of Alberta
Edmonton, AB, Canada
Presenting Author(s)
David Y.. Yang, MD1, Camille Lupianez-Merly, MD2, Kara Jencks, MD2, Saam Dilmaghani, MD, MPH2, Irene A. Busciglio, BS2, Deborah Eckert, RN2, Michael D. Ryks, 2, Roy Dyer, PhD2, Brady A. Vizenor, 2, Yuxi Zhao, PhD3, Anna Sapone, MD, PhD3, Michelle Rooks, PhD3, Jeremy Gale, PhD3, Michael Camilleri, MD, MACG2
1University of Alberta, Edmonton, AB, Canada; 2Mayo Clinic, Rochester, MN; 3Pfizer Research and Development, Cambridge, MA
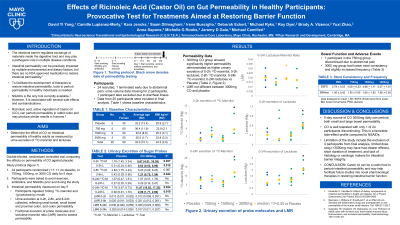
Introduction: Intestinal barrier dysfunction is implicated in multiple conditions. Tools to disrupt gut permeability in healthy participants are needed to facilitate research into agents that restore intestinal barrier integrity. Nonsteroidal anti-inflammatory drugs (NSAIDs) disrupt predominantly gastric and small intestinal permeability. Ricinoleic acid, the active component of castor oil (CO), increased colon permeability in rabbits (PMID 863205). The aim of this study was to determine the dose-related effects of CO on gut permeability in healthy human adults.
Methods: A single-center, double blinded, randomized controlled trial of healthy participants 18-70 y/o randomized (1:1:1:1) to placebo (PBO), or 750 mg, 1500 mg, 3000 mg CO administered orally daily for 5 days. On day 5, gut permeability was tested by quantifying urinary excretion of probe molecules after oral ingestion of 100 mg 13C-mannitol (13C-M) and 1 g lactulose (PMID 33865841). Three urine collections were acquired: 0-2, 2-8, and 8-24 hours. Bowel functions were appraised using a standard daily diary (stool consistency [BSFS], and bowel movement frequency). The primary endpoints were 2-8 h 13C-M and lactulose excretion, reflecting small intestinal and colonic permeability. Statistical analyses were completed in Sigmaplot using Kruskal-Wallis (KW) test for detecting differences among treatments and t-test for comparing 3000 mg CO to PBO.
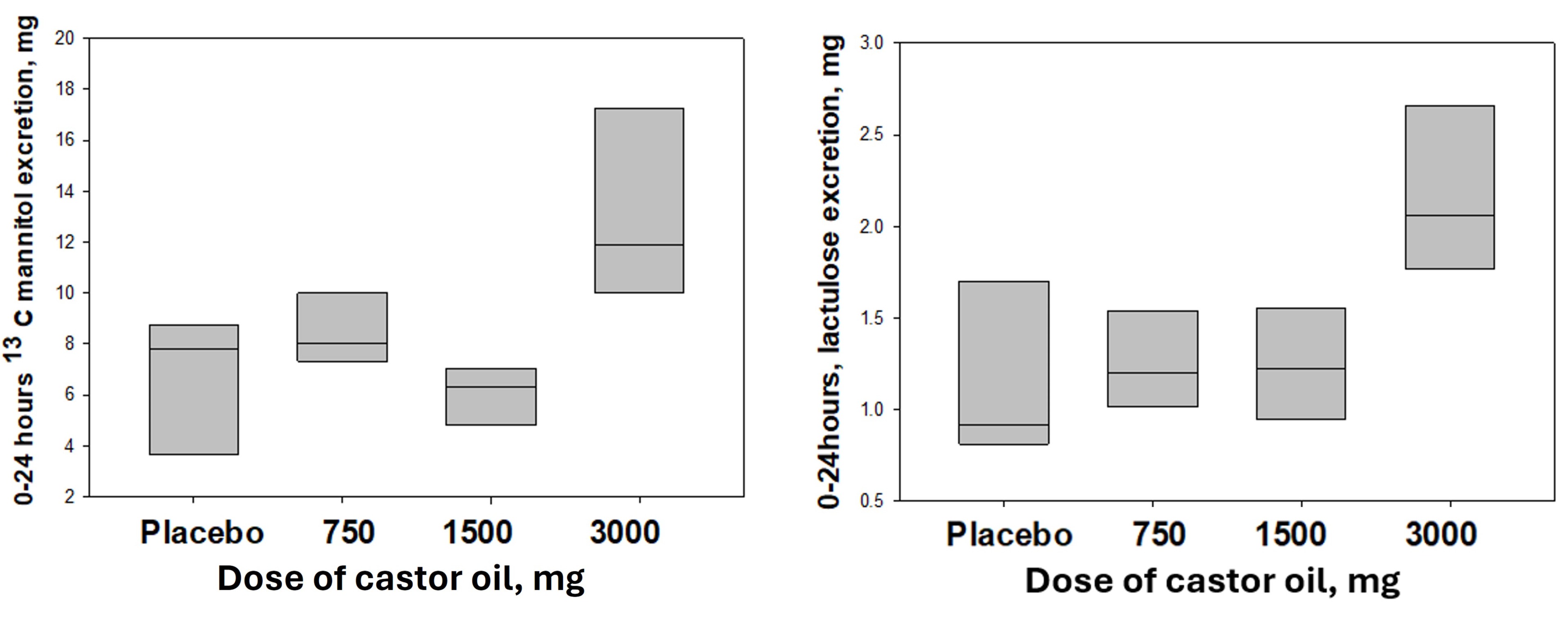
Results: 24 participants (12F) were recruited and 21 completed the trial (1 dropout, 2 incomplete data). Data from 1 participant were excluded due to recent suspected infectious gastroenteritis. Overall gut permeability assessed by 0-24 h excretion of the sugar probes (analyzed using KW test) showed significant differences among treatments for 13C-M (p=0.008), and borderline significant difference for lactulose (p=0.056) (table). For comparison of 3000 mg CO vs PBO by t-test, 13C-M (p=0.087) and lactulose (p=0.044) for 2-8 h urine were higher for 3000 mg CO group. 0-24 h urinary excretions in 3000 mg CO group were significantly greater than PBO group for both probes (table). In addition, 3000 mg CO was associated with looser stool consistency and slightly increased bowel frequency. Adverse events were generally mild with 1 drop-out due to abdominal pain.
Discussion: A 5-day course of 3,000 mg castor oil increases gut permeability in healthy participants, suggesting that castor oil can be an alternative to nonsteroidal anti-inflammatory drugs for studies requiring the perturbation of gut permeability.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
David Y.. Yang, MD1, Camille Lupianez-Merly, MD2, Kara Jencks, MD2, Saam Dilmaghani, MD, MPH2, Irene A. Busciglio, BS2, Deborah Eckert, RN2, Michael D. Ryks, 2, Roy Dyer, PhD2, Brady A. Vizenor, 2, Yuxi Zhao, PhD3, Anna Sapone, MD, PhD3, Michelle Rooks, PhD3, Jeremy Gale, PhD3, Michael Camilleri, MD, MACG2. P4042 - Dose-Related Effects of Ricinoleic Acid (Castor Oil) on Gut Permeability in Healthy Participants: Provocative Test for Treatments Aimed at Restoring Barrier Function, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Alberta, Edmonton, AB, Canada; 2Mayo Clinic, Rochester, MN; 3Pfizer Research and Development, Cambridge, MA
Introduction: Intestinal barrier dysfunction is implicated in multiple conditions. Tools to disrupt gut permeability in healthy participants are needed to facilitate research into agents that restore intestinal barrier integrity. Nonsteroidal anti-inflammatory drugs (NSAIDs) disrupt predominantly gastric and small intestinal permeability. Ricinoleic acid, the active component of castor oil (CO), increased colon permeability in rabbits (PMID 863205). The aim of this study was to determine the dose-related effects of CO on gut permeability in healthy human adults.
Methods: A single-center, double blinded, randomized controlled trial of healthy participants 18-70 y/o randomized (1:1:1:1) to placebo (PBO), or 750 mg, 1500 mg, 3000 mg CO administered orally daily for 5 days. On day 5, gut permeability was tested by quantifying urinary excretion of probe molecules after oral ingestion of 100 mg 13C-mannitol (13C-M) and 1 g lactulose (PMID 33865841). Three urine collections were acquired: 0-2, 2-8, and 8-24 hours. Bowel functions were appraised using a standard daily diary (stool consistency [BSFS], and bowel movement frequency). The primary endpoints were 2-8 h 13C-M and lactulose excretion, reflecting small intestinal and colonic permeability. Statistical analyses were completed in Sigmaplot using Kruskal-Wallis (KW) test for detecting differences among treatments and t-test for comparing 3000 mg CO to PBO.
Results: 24 participants (12F) were recruited and 21 completed the trial (1 dropout, 2 incomplete data). Data from 1 participant were excluded due to recent suspected infectious gastroenteritis. Overall gut permeability assessed by 0-24 h excretion of the sugar probes (analyzed using KW test) showed significant differences among treatments for 13C-M (p=0.008), and borderline significant difference for lactulose (p=0.056) (table). For comparison of 3000 mg CO vs PBO by t-test, 13C-M (p=0.087) and lactulose (p=0.044) for 2-8 h urine were higher for 3000 mg CO group. 0-24 h urinary excretions in 3000 mg CO group were significantly greater than PBO group for both probes (table). In addition, 3000 mg CO was associated with looser stool consistency and slightly increased bowel frequency. Adverse events were generally mild with 1 drop-out due to abdominal pain.
Discussion: A 5-day course of 3,000 mg castor oil increases gut permeability in healthy participants, suggesting that castor oil can be an alternative to nonsteroidal anti-inflammatory drugs for studies requiring the perturbation of gut permeability.

Figure: Figure. Urine excretion of sugar probes 0-24h on day 5 of Castor Oil treatment: Dose-related effects
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
David Yang indicated no relevant financial relationships.
Camille Lupianez-Merly indicated no relevant financial relationships.
Kara Jencks indicated no relevant financial relationships.
Saam Dilmaghani indicated no relevant financial relationships.
Irene Busciglio indicated no relevant financial relationships.
Deborah Eckert indicated no relevant financial relationships.
Michael Ryks indicated no relevant financial relationships.
Roy Dyer indicated no relevant financial relationships.
Brady Vizenor indicated no relevant financial relationships.
Yuxi Zhao: Pfizer – Employee, Stock-publicly held company(excluding mutual/index funds).
Anna Sapone: Pfizer – Employee, Stock-publicly held company(excluding mutual/index funds).
Michelle Rooks: Pfizer – Employee, Stock-publicly held company(excluding mutual/index funds).
Jeremy Gale: Pfizer – Employee, Stock-publicly held company(excluding mutual/index funds).
Michael Camilleri: Aclipse Therapeutics – Consultant. Aditum Bio – Consultant. Alfasigma – Consultant. APHAIA Pharma – Consultant. Bilayer Therapeutics – Stock Options. Biocodex – Grant/Research Support. BioKier – Consultant. Brightseed Bio – Consultant, Grant/Research Support. Coloplast – Consultant. Cosmo Pharmceuticals – Consultant. Dignify Therapeutics – Stock Options. Enterin – Stock Options. Invea Therapeutics – Consultant. Kallyope – Consultant. Medpace – Consultant. Monteresearch – Consultant. Neurogastrx – Consultant. NGM Biopharmaceuticals – Grant/Research Support. Pfizer – Grant/Research Support. Phenomix – Advisor or Review Panel Member, Stock Options. Renexxion – Consultant. SKYE Bioscience – Consultant. Sumitomo / Sunovion – Consultant. Synlogic – Consultant. Takeda – Consultant. Thelium Therapeutics – Stock Options. Vanda – Grant/Research Support.
David Y.. Yang, MD1, Camille Lupianez-Merly, MD2, Kara Jencks, MD2, Saam Dilmaghani, MD, MPH2, Irene A. Busciglio, BS2, Deborah Eckert, RN2, Michael D. Ryks, 2, Roy Dyer, PhD2, Brady A. Vizenor, 2, Yuxi Zhao, PhD3, Anna Sapone, MD, PhD3, Michelle Rooks, PhD3, Jeremy Gale, PhD3, Michael Camilleri, MD, MACG2. P4042 - Dose-Related Effects of Ricinoleic Acid (Castor Oil) on Gut Permeability in Healthy Participants: Provocative Test for Treatments Aimed at Restoring Barrier Function, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
