Tuesday Poster Session
Category: Functional Bowel Disease
P4043 - Evaluating Equity in Clinical Trial Accessibility: A Systematic Review of Demographic, Socioeconomic, and Educational Disparities in IBS Drug Trials
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- AK
Anthony Kerbage, MD
Cleveland Clinic
Cleveland, OH
Presenting Author(s)
Award: Presidential Poster Award
Anthony Kerbage, MD1, Jack Loesch, BA1, Eyad Hamza, MS1, Sulman Khan, DO1, Neil Nero, 1, Madison Simons, PhD1, Anthony J. Lembo, MD2
1Cleveland Clinic, Cleveland, OH; 2Digestive Disease Institute, Cleveland Clinic, Cleveland, OH
Introduction: Irritable Bowel Syndrome (IBS), a disorder of gut-brain interaction imposes a significant economic burden due to its high prevalence and the chronic nature of its symptoms. IBS currently has seven FDA-approved treatments. Despite efforts to improve diversity in randomized controlled trials (RCT) participation, significant disparities remain in various medical fields, yet these have not been thoroughly examined within the context of IBS. We aimed to investigate the demographic, socioeconomic, and educational disparities in IBS drug trials.
Methods: We conducted a systematic review on Phase III RCTs on FDA-approved drugs for the treatment of IBS with constipation (IBS-C) and IBS with diarrhea (IBS-D) in the United States. Data on participant demographics were systematically extracted and analyzed to identify disparities. We used clinicaltrials.gov to map the trial sites of each study, to understand the geographical spread and potential accessibility within the trials. Counties with at least one trial site were labeled as trial counties. For each county, we also defined geographically adjacent counties using the US Census Bureau 2010 County Adjacency file.
Results: Our analysis included 17 studies encompassing 21 trials with 17,428 participants. 77.3% of participants were female, with a mean age of 45.4 years. Race was reported in 95% of the trials, but only 35% disclosed ethnicity. A more detailed breakdown of race was provided in 28% of the trials. White participants constituted the majority at 79.3%. Hispanics accounted for only 5.9%. Counties without trial sites had smaller average population sizes compared to trial and trial-adjacent counties. Socioeconomic indicators such as poverty rates, median household income, educational attainment, and broadband internet access were lower in counties without trial sites, which had higher average Area Deprivation Index scores indicating greater deprivation.
Discussion: Our findings reveal significant disparities in participation in IBS RCTs across racial, ethnic, gender, and geographic lines. This may compromise the generalizability of trial results and reflect broader issues of equity within healthcare research. Addressing these gaps requires a concerted effort to enhance trial design and recruitment strategies, ensuring that research on IBS is accessible and representative of the diverse patient population it aims to serve, ultimately leading to more equitable healthcare outcomes for all individuals affected by IBS.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Anthony Kerbage, MD1, Jack Loesch, BA1, Eyad Hamza, MS1, Sulman Khan, DO1, Neil Nero, 1, Madison Simons, PhD1, Anthony J. Lembo, MD2. P4043 - Evaluating Equity in Clinical Trial Accessibility: A Systematic Review of Demographic, Socioeconomic, and Educational Disparities in IBS Drug Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Anthony Kerbage, MD1, Jack Loesch, BA1, Eyad Hamza, MS1, Sulman Khan, DO1, Neil Nero, 1, Madison Simons, PhD1, Anthony J. Lembo, MD2
1Cleveland Clinic, Cleveland, OH; 2Digestive Disease Institute, Cleveland Clinic, Cleveland, OH
Introduction: Irritable Bowel Syndrome (IBS), a disorder of gut-brain interaction imposes a significant economic burden due to its high prevalence and the chronic nature of its symptoms. IBS currently has seven FDA-approved treatments. Despite efforts to improve diversity in randomized controlled trials (RCT) participation, significant disparities remain in various medical fields, yet these have not been thoroughly examined within the context of IBS. We aimed to investigate the demographic, socioeconomic, and educational disparities in IBS drug trials.
Methods: We conducted a systematic review on Phase III RCTs on FDA-approved drugs for the treatment of IBS with constipation (IBS-C) and IBS with diarrhea (IBS-D) in the United States. Data on participant demographics were systematically extracted and analyzed to identify disparities. We used clinicaltrials.gov to map the trial sites of each study, to understand the geographical spread and potential accessibility within the trials. Counties with at least one trial site were labeled as trial counties. For each county, we also defined geographically adjacent counties using the US Census Bureau 2010 County Adjacency file.
Results: Our analysis included 17 studies encompassing 21 trials with 17,428 participants. 77.3% of participants were female, with a mean age of 45.4 years. Race was reported in 95% of the trials, but only 35% disclosed ethnicity. A more detailed breakdown of race was provided in 28% of the trials. White participants constituted the majority at 79.3%. Hispanics accounted for only 5.9%. Counties without trial sites had smaller average population sizes compared to trial and trial-adjacent counties. Socioeconomic indicators such as poverty rates, median household income, educational attainment, and broadband internet access were lower in counties without trial sites, which had higher average Area Deprivation Index scores indicating greater deprivation.
Discussion: Our findings reveal significant disparities in participation in IBS RCTs across racial, ethnic, gender, and geographic lines. This may compromise the generalizability of trial results and reflect broader issues of equity within healthcare research. Addressing these gaps requires a concerted effort to enhance trial design and recruitment strategies, ensuring that research on IBS is accessible and representative of the diverse patient population it aims to serve, ultimately leading to more equitable healthcare outcomes for all individuals affected by IBS.

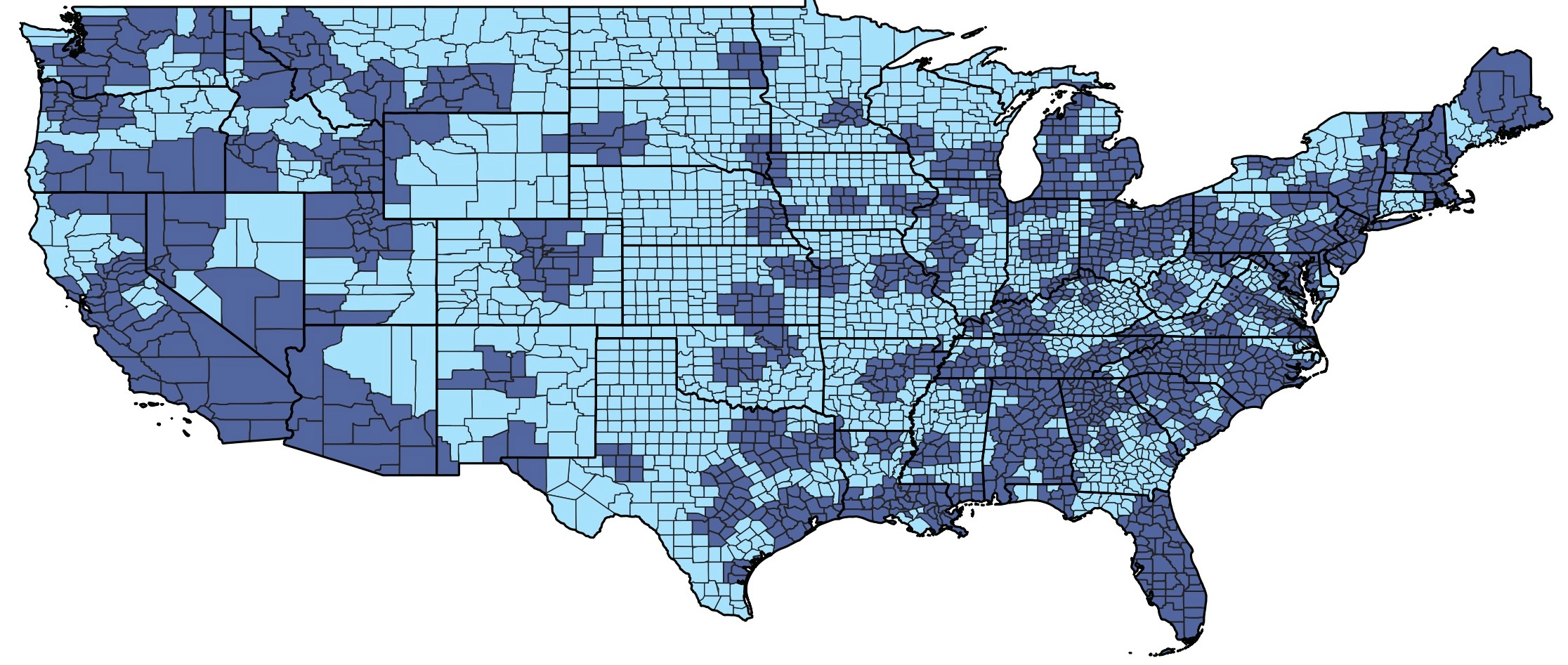
Figure: Figure 2. U.S. County Map Indicating Geographic Distribution of Clinical Trial Sites
Dark blue: Trial and trial-adjacent counties
Light blue: Counties without a trial site
Hawaii and Alaska are not included due to no clinical trial sites.
Dark blue: Trial and trial-adjacent counties
Light blue: Counties without a trial site
Hawaii and Alaska are not included due to no clinical trial sites.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Anthony Kerbage indicated no relevant financial relationships.
Jack Loesch: Eli Lilly and Company – Stock-publicly held company(excluding mutual/index funds).
Eyad Hamza indicated no relevant financial relationships.
Sulman Khan indicated no relevant financial relationships.
Neil Nero indicated no relevant financial relationships.
Madison Simons indicated no relevant financial relationships.
Anthony Lembo: Allurion – Stock Options. Bristol Myers Squibb – Stock Options. Johnson & Johnson – Stock Options. Vibrant Advisory Board – Advisory Committee/Board Member.
Anthony Kerbage, MD1, Jack Loesch, BA1, Eyad Hamza, MS1, Sulman Khan, DO1, Neil Nero, 1, Madison Simons, PhD1, Anthony J. Lembo, MD2. P4043 - Evaluating Equity in Clinical Trial Accessibility: A Systematic Review of Demographic, Socioeconomic, and Educational Disparities in IBS Drug Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


