Tuesday Poster Session
Category: Functional Bowel Disease
P4073 - Impact of Weight on the Efficacy, Time to Response, and Safety of Linaclotide in Adults With Chronic Idiopathic Constipation or Irritable Bowel Syndrome With Constipation: Post Hoc Descriptive Analysis by Body Mass Index
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Satish S. C. Rao, MD, PhD
Medical College of Georgia, Augusta University
Augusta, GA
Presenting Author(s)
Baharak Moshiree, MD1, Anthony J. Lembo, MD2, Brooks D. Cash, MD, FACG3, Wendy Chen, PharmD4, Valentina Shakhnovich, MD5, James Wu, PhD5, Moming Li, PhD4, Satish S. C.. Rao, MD, PhD6
1Advocate Health Wake Forest Medical University, Charlotte, NC; 2Digestive Disease Institute, Cleveland Clinic, Cleveland, OH; 3The University of Texas Health Science Center at Houston, Houston, TX; 4AbbVie, Inc., North Chicago, IL; 5Ironwood Pharmaceuticals, Inc., Boston, MA; 6Medical College of Georgia, Augusta University, Augusta, GA
Introduction: Previous research has suggested that constipation may be more prevalent in adults with an overweight or obese BMI than those with a normal weight BMI. However, there are limited data on the impact of BMI on treatment responses in adults with constipation and it is unclear whether adjustments to standard dosing of medications are needed. This post hoc analysis evaluated the efficacy and safety of LIN in adults with chronic idiopathic constipation (CIC) or irritable bowel syndrome with constipation (IBS-C) by BMI category.
Methods: Data from 7 Phase 3 randomized studies of adults who met modified Rome II/III criteria for CIC or IBS-C and received FDA-approved doses of LIN (CIC 72 µg or 145 µg; IBS-C 290 µg) or placebo (PBO) were pooled and stratified by BMI category (normal weight 18.5 to < 25 kg/m2; overweight 25 to < 30 kg/m2; obese ≥30 kg/m2). Patients with a BMI < 18.5 kg/m2 were excluded. Primary efficacy endpoints were the complete spontaneous bowel movement (CSBM) overall responder rate for CIC patients (CSBM weekly rate ≥3 and ≥baseline+1 for ≥9/12 weeks) and the abdominal pain and constipation (APC)+1 responder rate for IBS-C patients (≥30% reduction in abdominal pain score and CSBM weekly rate ≥baseline+1 for ≥6/12 weeks). Change from baseline over 12 weeks in CIC or IBS-C symptoms, time to change from baseline in CSBM weekly rate ≥1 and safety were also assessed.
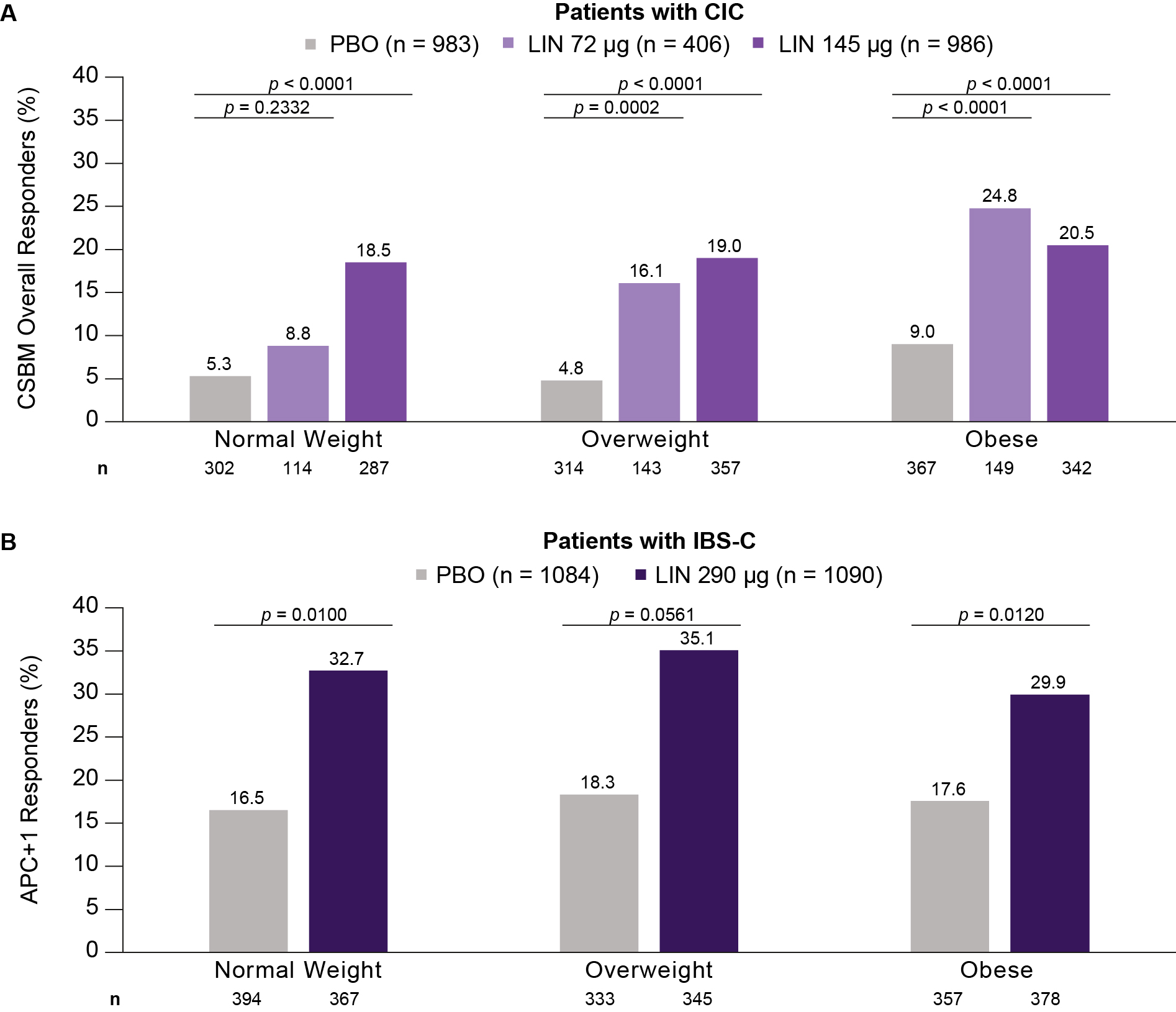
Results: The analysis included 2375 CIC and 2174 IBS-C patients (n: normal weight 703 and 761; overweight 814 and 678; obese 858 and 735, respectively). Baseline characteristics were similar across BMI subgroups. The proportion of CSBM overall responders was higher for LIN than PBO across subgroups, with greater treatment effects seen in CIC patients with overweight or obesity vs those with normal weight. For IBS-C patients, the proportion of APC+1 responders was higher for LIN than PBO across subgroups (Figure). Improvements at 12 weeks in several CIC or IBS-C symptoms were greater with LIN than PBO across BMI subgroups (Table). The median time to change from baseline in CSBM weekly rate ≥1 was similar across subgroups and improved with LIN (1–3 weeks) vs PBO (4–5 weeks), except for CIC patients with normal weight who had LIN 72 μg (4 weeks). The frequency of TEAEs was similar across subgroups in CIC and IBS-C.
Discussion: LIN improved bowel symptoms in patients with CIC and IBS-C and was similarly efficacious in those with normal weight, overweight or obesity. TEAEs were consistent with the known safety profile of LIN.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Baharak Moshiree, MD1, Anthony J. Lembo, MD2, Brooks D. Cash, MD, FACG3, Wendy Chen, PharmD4, Valentina Shakhnovich, MD5, James Wu, PhD5, Moming Li, PhD4, Satish S. C.. Rao, MD, PhD6. P4073 - Impact of Weight on the Efficacy, Time to Response, and Safety of Linaclotide in Adults With Chronic Idiopathic Constipation or Irritable Bowel Syndrome With Constipation: Post Hoc Descriptive Analysis by Body Mass Index, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Advocate Health Wake Forest Medical University, Charlotte, NC; 2Digestive Disease Institute, Cleveland Clinic, Cleveland, OH; 3The University of Texas Health Science Center at Houston, Houston, TX; 4AbbVie, Inc., North Chicago, IL; 5Ironwood Pharmaceuticals, Inc., Boston, MA; 6Medical College of Georgia, Augusta University, Augusta, GA
Introduction: Previous research has suggested that constipation may be more prevalent in adults with an overweight or obese BMI than those with a normal weight BMI. However, there are limited data on the impact of BMI on treatment responses in adults with constipation and it is unclear whether adjustments to standard dosing of medications are needed. This post hoc analysis evaluated the efficacy and safety of LIN in adults with chronic idiopathic constipation (CIC) or irritable bowel syndrome with constipation (IBS-C) by BMI category.
Methods: Data from 7 Phase 3 randomized studies of adults who met modified Rome II/III criteria for CIC or IBS-C and received FDA-approved doses of LIN (CIC 72 µg or 145 µg; IBS-C 290 µg) or placebo (PBO) were pooled and stratified by BMI category (normal weight 18.5 to < 25 kg/m2; overweight 25 to < 30 kg/m2; obese ≥30 kg/m2). Patients with a BMI < 18.5 kg/m2 were excluded. Primary efficacy endpoints were the complete spontaneous bowel movement (CSBM) overall responder rate for CIC patients (CSBM weekly rate ≥3 and ≥baseline+1 for ≥9/12 weeks) and the abdominal pain and constipation (APC)+1 responder rate for IBS-C patients (≥30% reduction in abdominal pain score and CSBM weekly rate ≥baseline+1 for ≥6/12 weeks). Change from baseline over 12 weeks in CIC or IBS-C symptoms, time to change from baseline in CSBM weekly rate ≥1 and safety were also assessed.
Results: The analysis included 2375 CIC and 2174 IBS-C patients (n: normal weight 703 and 761; overweight 814 and 678; obese 858 and 735, respectively). Baseline characteristics were similar across BMI subgroups. The proportion of CSBM overall responders was higher for LIN than PBO across subgroups, with greater treatment effects seen in CIC patients with overweight or obesity vs those with normal weight. For IBS-C patients, the proportion of APC+1 responders was higher for LIN than PBO across subgroups (Figure). Improvements at 12 weeks in several CIC or IBS-C symptoms were greater with LIN than PBO across BMI subgroups (Table). The median time to change from baseline in CSBM weekly rate ≥1 was similar across subgroups and improved with LIN (1–3 weeks) vs PBO (4–5 weeks), except for CIC patients with normal weight who had LIN 72 μg (4 weeks). The frequency of TEAEs was similar across subgroups in CIC and IBS-C.
Discussion: LIN improved bowel symptoms in patients with CIC and IBS-C and was similarly efficacious in those with normal weight, overweight or obesity. TEAEs were consistent with the known safety profile of LIN.

Figure: Proportion of A) CSBM Overall Responders for Patients with CIC and B) APC+1 Responders for Patients With IBS-C by BMI Category.
n represents the number of patients with responder data available within each group. p-values are descriptive only and show the difference in responder rate obtained from Cochran-Mantel-Haenszel tests controlling for geographic region for pairwise comparisons of each LIN dose vs PBO. A CSBM overall responder was defined as a patient with ≥3 CSBMs per week and an increase of ≥1 CSBM per week from baseline for ≥9 of the 12 treatment-period weeks. An APC+1 responder was defined as a patient with a ≥30% reduction in abdominal pain score and an increase of ≥1 CSBM per week from baseline for ≥6 of the 12 treatment-period weeks.
APC, abdominal pain and constipation; CIC, chronic idiopathic constipation; CSBM, complete spontaneous bowel movement; IBS-C, irritable bowel syndrome with constipation; LIN, linaclotide; PBO, placebo.
n represents the number of patients with responder data available within each group. p-values are descriptive only and show the difference in responder rate obtained from Cochran-Mantel-Haenszel tests controlling for geographic region for pairwise comparisons of each LIN dose vs PBO. A CSBM overall responder was defined as a patient with ≥3 CSBMs per week and an increase of ≥1 CSBM per week from baseline for ≥9 of the 12 treatment-period weeks. An APC+1 responder was defined as a patient with a ≥30% reduction in abdominal pain score and an increase of ≥1 CSBM per week from baseline for ≥6 of the 12 treatment-period weeks.
APC, abdominal pain and constipation; CIC, chronic idiopathic constipation; CSBM, complete spontaneous bowel movement; IBS-C, irritable bowel syndrome with constipation; LIN, linaclotide; PBO, placebo.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Baharak Moshiree: AbbVie – Advisory Committee/Board Member. Allergan – Advisory Committee/Board Member. Alnylam Pharmaceuticals – Advisory Committee/Board Member. Ardelyx – Advisory Committee/Board Member. Atmo Biosciences – Advisory Committee/Board Member, Grant/Research Support. Biora Therapeutics (formerly Progenity) – Advisory Committee/Board Member. CinDome Pharma – Grant/Research Support. CinPhloro Pharma – Grant/Research Support. Restalsis Health – Grant/Research Support. Salix Pharmaceuticals of Bausch Health – Grant/Research Support. Takeda Pharmaceuticals – Grant/Research Support.
Anthony Lembo: Allurion – Stock Options. Bristol Myers Squibb – Stock Options. Johnson & Johnson – Stock Options. Vibrant Advisory Board – Advisory Committee/Board Member.
Brooks Cash: AbbVie – Consultant, Speaker. Alnylam Pharmaceuticals – Speaker. Ardelyx – Consultant, Speaker. AstraZeneca – Consultant, Speaker. Phathom Pharmaceuticals – Consultant, Speaker. QOL Medical LLC – Speaker. Regeneron Pharmaceuticals – Speaker. Salix Pharmaceuticals – Consultant, Speaker. Sanofi – Speaker.
Wendy Chen: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Valentina Shakhnovich: Ironwood Pharmaceuticals – Employee, Stock-publicly held company(excluding mutual/index funds).
James Wu: Ironwood Pharmaceuticals – Employee, Stock-publicly held company(excluding mutual/index funds).
Moming Li: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Satish Rao: Ironwood Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support. Pallette life sciences – Advisor or Review Panel Member.
Baharak Moshiree, MD1, Anthony J. Lembo, MD2, Brooks D. Cash, MD, FACG3, Wendy Chen, PharmD4, Valentina Shakhnovich, MD5, James Wu, PhD5, Moming Li, PhD4, Satish S. C.. Rao, MD, PhD6. P4073 - Impact of Weight on the Efficacy, Time to Response, and Safety of Linaclotide in Adults With Chronic Idiopathic Constipation or Irritable Bowel Syndrome With Constipation: Post Hoc Descriptive Analysis by Body Mass Index, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
