Tuesday Poster Session
Category: Functional Bowel Disease
P4074 - How Valuable is an Electronic Stool Diary App in the Management of Chronic Idiopathic Constipation (CIC) When Using the Vibrating Capsule (VC)
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Satish S. C. Rao, MD, PhD
Medical College of Georgia, Augusta University
Augusta, GA
Presenting Author(s)
Satish S. C.. Rao, MD, PhD1, Darren M.. Brenner, MD2, Brooks D. Cash, MD, FACG3, Lin Chang, MD4, William D. Chey, MD, FACG5, Bryan Curtin, MD6, Christine Frissora, MD7, Anthony J. Lembo, MD8, Linda Nguyen, MD9, Brennan Spiegel, MD, MSHS10, Eamonn Quigley, MD11
1Medical College of Georgia, Augusta University, Augusta, GA; 2Northwestern University, Chicago, IL; 3McGovern Medical School at UTHealth, Houston, TX; 4Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA, Los Angeles, CA; 5University of Michigan, Ann Arbor, MI; 6Mercy Memorial Hospital, Baltimore, MD; 7Weill Cornell Medical College, New York, NY; 8Digestive Disease Institute, Cleveland Clinic, Cleveland, OH; 9Stanford University School of Medicine, Stanford, CA; 10Cedars-Sinai Medical Center, Los Angeles, CA; 11Houston Methodist Hospital, Houston, TX
Introduction: Approximately 60% of CIC patients express dissatisfaction with current treatments. Furthermore, there is paucity of objective tools to monitor patient's symptoms and/or compliance with prescribed therapies. To determine if feedback received through a daily electronic stool diary application (app) along with treatment reminders led to improvement in bowel symptoms and improved compliance with therapy in patients with CIC using the VC, an FDA approved treatment.
Methods: In a post-marketing study, CIC patients who were prescribed VC (activation pod + 20 capsules for one month), were also asked to use an app to record bowel symptoms, bowel movement (BM) frequency, complete spontaneous BM (CSBM), stool consistency (Bristol Stool Scale 1-7), straining effort (scale of 1 [none] to 4 [severe] and time spent on toilet. Data from the first week of treatment (baseline) were compared with changes during subsequent weeks while receiving treatment. Patients using stool app were compared to those who did not.
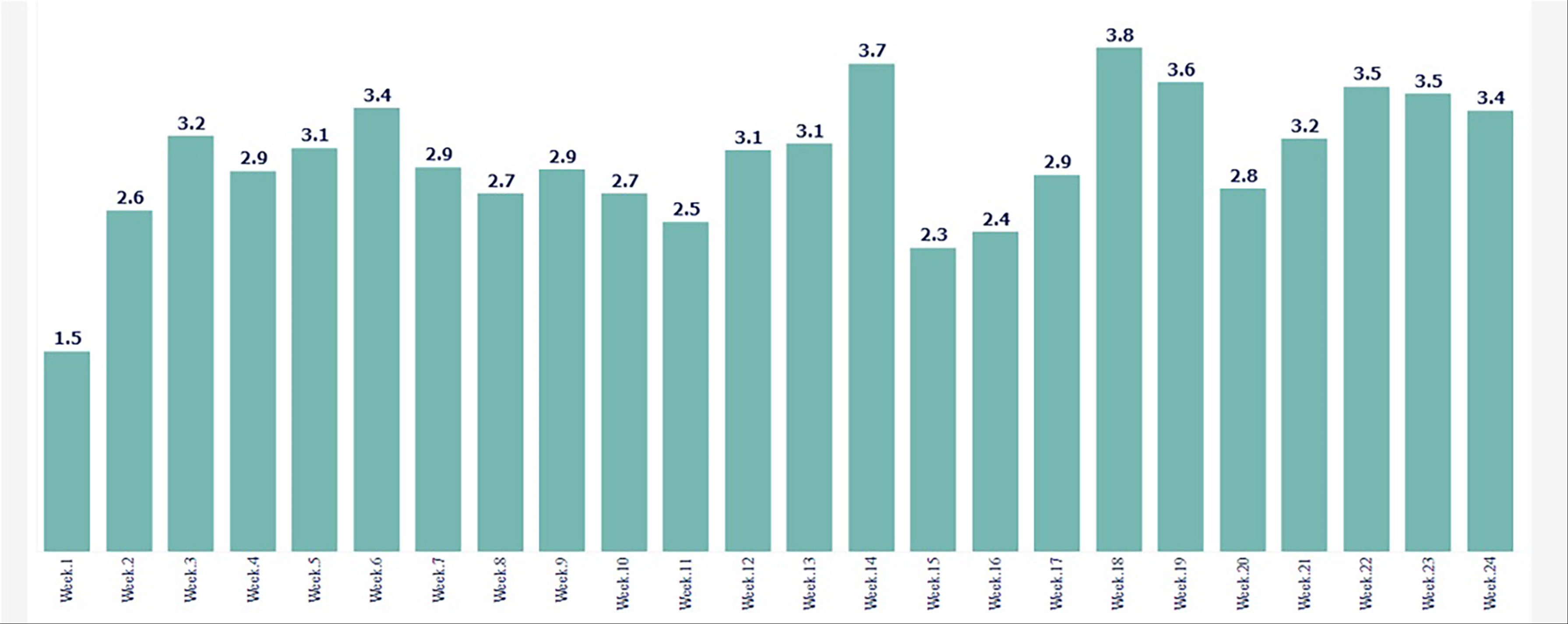
Results: From February to November 2023, 1075 patients (82.5% female) received VC treatment, of whom 453 (42%) downloaded the app and used the VC ≥ one week (baseline), Table 1. Proportionately more women than men and younger patients used the app, but interestingly 36% of patients ≥ 65 years old also engaged with the app. 58 (12.8%) patients used the app for at least 8 weeks of treatment. Patients who used the app (68%) were twice as likely to order a first refill compared to those who did not use it (34%) (P< 0.0001). App users had a significantly increase in the number of CSBM/week, with a more than two-fold increase in CSBM rate (p< 0.0002) Figure. Also, the mean stool consistency improved from 3.3 (baseline) to 4.3 (treatment), (p< 0.0001), and the mean straining effort decreased significantly from 3.0 to 1.6 (p< 0.0001), and the time spent on toilet decreased from 33 minutes per CSBM to 15 minutes (p< 0.0001).
Discussion: In this community-based, novel, observational study, patients who took VC and kept a daily electronic stool diary app had significantly higher first reorder rates and demonstrated significant improvements in key constipation symptoms. It is possible that positive feedback reinforced by the e-diary app together with relevant treatment reminders may have contributed to the increased compliance with therapy resulting in improved treatment efficacy. An e-diary app may provide incremental benefit and facilitate treatment monitoring CIC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Satish S. C.. Rao, MD, PhD1, Darren M.. Brenner, MD2, Brooks D. Cash, MD, FACG3, Lin Chang, MD4, William D. Chey, MD, FACG5, Bryan Curtin, MD6, Christine Frissora, MD7, Anthony J. Lembo, MD8, Linda Nguyen, MD9, Brennan Spiegel, MD, MSHS10, Eamonn Quigley, MD11. P4074 - How Valuable is an Electronic Stool Diary App in the Management of Chronic Idiopathic Constipation (CIC) When Using the Vibrating Capsule (VC), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Medical College of Georgia, Augusta University, Augusta, GA; 2Northwestern University, Chicago, IL; 3McGovern Medical School at UTHealth, Houston, TX; 4Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA, Los Angeles, CA; 5University of Michigan, Ann Arbor, MI; 6Mercy Memorial Hospital, Baltimore, MD; 7Weill Cornell Medical College, New York, NY; 8Digestive Disease Institute, Cleveland Clinic, Cleveland, OH; 9Stanford University School of Medicine, Stanford, CA; 10Cedars-Sinai Medical Center, Los Angeles, CA; 11Houston Methodist Hospital, Houston, TX
Introduction: Approximately 60% of CIC patients express dissatisfaction with current treatments. Furthermore, there is paucity of objective tools to monitor patient's symptoms and/or compliance with prescribed therapies. To determine if feedback received through a daily electronic stool diary application (app) along with treatment reminders led to improvement in bowel symptoms and improved compliance with therapy in patients with CIC using the VC, an FDA approved treatment.
Methods: In a post-marketing study, CIC patients who were prescribed VC (activation pod + 20 capsules for one month), were also asked to use an app to record bowel symptoms, bowel movement (BM) frequency, complete spontaneous BM (CSBM), stool consistency (Bristol Stool Scale 1-7), straining effort (scale of 1 [none] to 4 [severe] and time spent on toilet. Data from the first week of treatment (baseline) were compared with changes during subsequent weeks while receiving treatment. Patients using stool app were compared to those who did not.
Results: From February to November 2023, 1075 patients (82.5% female) received VC treatment, of whom 453 (42%) downloaded the app and used the VC ≥ one week (baseline), Table 1. Proportionately more women than men and younger patients used the app, but interestingly 36% of patients ≥ 65 years old also engaged with the app. 58 (12.8%) patients used the app for at least 8 weeks of treatment. Patients who used the app (68%) were twice as likely to order a first refill compared to those who did not use it (34%) (P< 0.0001). App users had a significantly increase in the number of CSBM/week, with a more than two-fold increase in CSBM rate (p< 0.0002) Figure. Also, the mean stool consistency improved from 3.3 (baseline) to 4.3 (treatment), (p< 0.0001), and the mean straining effort decreased significantly from 3.0 to 1.6 (p< 0.0001), and the time spent on toilet decreased from 33 minutes per CSBM to 15 minutes (p< 0.0001).
Discussion: In this community-based, novel, observational study, patients who took VC and kept a daily electronic stool diary app had significantly higher first reorder rates and demonstrated significant improvements in key constipation symptoms. It is possible that positive feedback reinforced by the e-diary app together with relevant treatment reminders may have contributed to the increased compliance with therapy resulting in improved treatment efficacy. An e-diary app may provide incremental benefit and facilitate treatment monitoring CIC.

Figure: Mean number of CSBM/ week during treatment with vibrating capsule in app users
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Satish Rao: Ironwood Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support. Pallette life sciences – Advisor or Review Panel Member.
Darren Brenner: AbbVie – Consultant, Speaker. Anji Pharmaceuticals – Consultant. Ardelyx – Advisor or Review Panel Member, Consultant, Speaker. Bayer – Consultant. Blueprint Medicines – Advisor or Review Panel Member. CinPhloro – Advisor or Review Panel Member, Consultant. Dr. Reddy's Laboratories – Consultant. Entrinsic Bioscience – Consultant. Gemelli Biotech – Advisor or Review Panel Member, Consultant. Ironwood Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Laborie – Advisor or Review Panel Member. Mahana Therapeutics – Advisor or Review Panel Member, Consultant. Owlstone Medical – Advisor or Review Panel Member, Consultant, Stock Options. Salix Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Vibrant Gastro – Advisor or Review Panel Member, Consultant.
Brooks Cash: Abbvie – Consultant, Speakers Bureau. Alnylam – Speakers Bureau. Ardelyx – Consultant, Speakers Bureau. Astra Zeneca – Consultant, Speakers Bureau. Phathom – Consultant, Speakers Bureau. QOL – Speakers Bureau. Salix – Speakers Bureau. Vibrant Advisory Board – Advisory Committee/Board Member.
Lin Chang: AbbVie – Speaker. AnX Robotica – Grant/Research Support. Ardelyx – Advisory Committee/Board Member. Arena Pharmaceuticals – Grant/Research Support. Atmo Biosciences – Advisory Committee/Board Member. Bausch Health – Consultant, Speaker. FoodMarble Digestive Health – Consultant, Stock Options. Ironwood Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support. ModifyHealth – Stock Options. Trellus Health – Consultant, Stock-publicly held company(excluding mutual/index funds). Vibrant Advisory Board – Advisory Committee/Board Member. Vibrant Gastro – Advisory Committee/Board Member.
William Chey: AbbVie – Consultant. Ardelyx – Consultant. Atmo BioSciences – Consultant. Comvita – Consultant. Corprata – Stock Options. Dieta Health – Stock Options. FoodMarble Digestive Health – Consultant, Stock Options. Ironwood Pharmaceuticals – Consultant. Modify Health – Stock Options. Nestlé S.A. – Consultant. Phathom Pharmaceuticals – Consultant. Redhill Biopharma – Consultant. Salix Pharmaceuticals – Consultant. Takeda – Consultant. Vibrant Gastro – Consultant.
Bryan Curtin: Vibrant Advisory Board – Advisory Committee/Board Member.
Christine Frissora: Vibrant Advisory Board – Advisory Committee/Board Member.
Anthony Lembo: Allurion – Stock Options. Bristol Myers Squibb – Stock Options. Johnson & Johnson – Stock Options. Vibrant Advisory Board – Advisory Committee/Board Member.
Linda Nguyen: Vibrant Advisory Board – Advisory Committee/Board Member.
Brennan Spiegel: Freenome – Grant/Research Support. Vibrant Advisory Board – Advisory Committee/Board Member.
Eamonn Quigley: Atmo Biosciences – Advisor or Review Panel Member, Consultant, Grant/Research Support. Cindome – Grant/Research Support. Food Marble – Consultant. Nimble – Consultant. Novozymes – Consultant. Takeda – Grant/Research Support. Vibrant – Advisor or Review Panel Member, Consultant, Grant/Research Support. Vibrant Advisory Board – Advisory Committee/Board Member.
Satish S. C.. Rao, MD, PhD1, Darren M.. Brenner, MD2, Brooks D. Cash, MD, FACG3, Lin Chang, MD4, William D. Chey, MD, FACG5, Bryan Curtin, MD6, Christine Frissora, MD7, Anthony J. Lembo, MD8, Linda Nguyen, MD9, Brennan Spiegel, MD, MSHS10, Eamonn Quigley, MD11. P4074 - How Valuable is an Electronic Stool Diary App in the Management of Chronic Idiopathic Constipation (CIC) When Using the Vibrating Capsule (VC), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
