Tuesday Poster Session
Category: General Endoscopy
P4094 - Decrease Risk of Intubation and Aspiration Pneumonia Associated with Endoscopic Procedure Among Patients on Glucagon-Like Peptide 1 Receptor Agonists
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Khaled Alsabbagh Alchirazi, MD
Aurora Healthcare
Brookfield, WI
Presenting Author(s)
Khaled Alsabbagh Alchirazi, MD1, Michelle Baliss, DO2, Ahmed El Telbany, MD, MPH3, Wissam Kiwan, MD4, Mohammad Bilal, MD5
1Aurora Healthcare, Brookfield, WI; 2SSM Health Saint Louis University Hospital, St. Louis, MO; 3University of New Mexico, Albuquerque, NM; 4Saint Louis University, St. Louis, MO; 5University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are commonly used to treat type 2 diabetes mellitus (T2DM) and obesity, have been linked with slowing the gut motility and potentially increasing gastric content retention. However, there is limited data of GLP-1 RAs on the risks of aspiration pneumonia and emergent endotracheal intubation in those undergoing esophagogastroduodenoscopy (EGD) or colonoscopy. Our study aims to explore the impact of GLP-1 RAs on the safety and outcomes of these endoscopic procedures.
Methods: We conducted a five-year study utilizing TriNetX data from 2020-2023 to examine adult patients undergoing EGD and colonoscopy. We compared outcomes between patients receiving GLP-1RAs and those not on these medications. To ensure comparability, groups were propensity score matched (PSM) based on age, gender, race, ethnicity, Charlson comorbidity index, body mass index, diabetes and sedation related medications and confounders that could affect gut motility or aspiration risks, table 1. Outcomes were assessed within 7-days and 30-days of EGD or colonoscopy. We utilized adjusted hazard ratios (HR) with 95% confidence intervals (CI) and Two-sided p-values < 0.05 was considered statistically significant
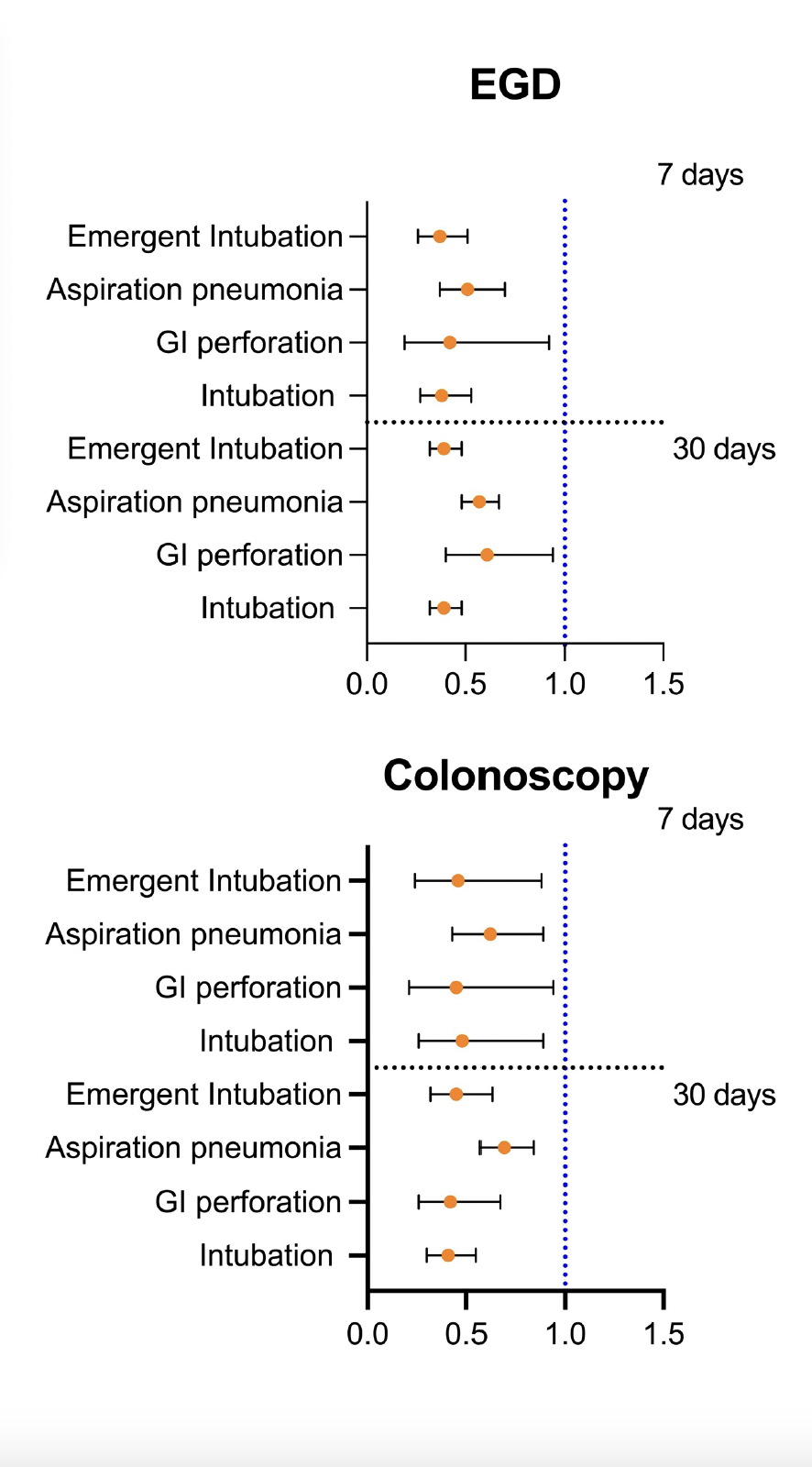
Results: After PSM, we found 69,618 patients who underwent EGD and 104,782 who underwent colonoscopy in both cohorts. Within 7 days of the EGD, patients with prior GLP-1 RAs use were at lower risk of emergent intubation (HR 0.37, 95% CI [0.26, 0.51]) and aspiration pneumonia (HR 0.51, [(0.37, 0.70]) compared to patients not receiving GLP-1RAs. Similar trends were observed within 7 days of colonoscopy between the two groups with (HR 0.46, [0.24, 0.88]) for emergent intubation; (HR 0.62, [0.43, 0.89]) for aspiration pneumonia; and (HR 0.45, [0.21, 0.94]) for GI perforation. Consistent findings were observed within 30 days of either EGD or colonoscopy between the two cohorts, Figure 1.
Discussion: While our study is limited by the retrospective design and inherent limitations of the database which does not account for medication adherence or cessation prior to endoscopic procedures, our results show that GLP-1RAs do not increase risk of aspiration or intubation in patients undergoing EGD and colonoscopy. Further prospective studies are needed to guide management of GLP-1RAs in the pre-operative period prior to endoscopic procedures.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Khaled Alsabbagh Alchirazi, MD1, Michelle Baliss, DO2, Ahmed El Telbany, MD, MPH3, Wissam Kiwan, MD4, Mohammad Bilal, MD5. P4094 - Decrease Risk of Intubation and Aspiration Pneumonia Associated with Endoscopic Procedure Among Patients on Glucagon-Like Peptide 1 Receptor Agonists, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Aurora Healthcare, Brookfield, WI; 2SSM Health Saint Louis University Hospital, St. Louis, MO; 3University of New Mexico, Albuquerque, NM; 4Saint Louis University, St. Louis, MO; 5University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are commonly used to treat type 2 diabetes mellitus (T2DM) and obesity, have been linked with slowing the gut motility and potentially increasing gastric content retention. However, there is limited data of GLP-1 RAs on the risks of aspiration pneumonia and emergent endotracheal intubation in those undergoing esophagogastroduodenoscopy (EGD) or colonoscopy. Our study aims to explore the impact of GLP-1 RAs on the safety and outcomes of these endoscopic procedures.
Methods: We conducted a five-year study utilizing TriNetX data from 2020-2023 to examine adult patients undergoing EGD and colonoscopy. We compared outcomes between patients receiving GLP-1RAs and those not on these medications. To ensure comparability, groups were propensity score matched (PSM) based on age, gender, race, ethnicity, Charlson comorbidity index, body mass index, diabetes and sedation related medications and confounders that could affect gut motility or aspiration risks, table 1. Outcomes were assessed within 7-days and 30-days of EGD or colonoscopy. We utilized adjusted hazard ratios (HR) with 95% confidence intervals (CI) and Two-sided p-values < 0.05 was considered statistically significant
Results: After PSM, we found 69,618 patients who underwent EGD and 104,782 who underwent colonoscopy in both cohorts. Within 7 days of the EGD, patients with prior GLP-1 RAs use were at lower risk of emergent intubation (HR 0.37, 95% CI [0.26, 0.51]) and aspiration pneumonia (HR 0.51, [(0.37, 0.70]) compared to patients not receiving GLP-1RAs. Similar trends were observed within 7 days of colonoscopy between the two groups with (HR 0.46, [0.24, 0.88]) for emergent intubation; (HR 0.62, [0.43, 0.89]) for aspiration pneumonia; and (HR 0.45, [0.21, 0.94]) for GI perforation. Consistent findings were observed within 30 days of either EGD or colonoscopy between the two cohorts, Figure 1.
Discussion: While our study is limited by the retrospective design and inherent limitations of the database which does not account for medication adherence or cessation prior to endoscopic procedures, our results show that GLP-1RAs do not increase risk of aspiration or intubation in patients undergoing EGD and colonoscopy. Further prospective studies are needed to guide management of GLP-1RAs in the pre-operative period prior to endoscopic procedures.

Figure: Figure 1: Outcomes Associated With Endoscopic Procedures in Glucagon-like Peptide 1 Receptor Agonist Users Compared to Nonusers within 7 and 30 days from EGD and Colonoscopy After Propensity Score Matching.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Khaled Alsabbagh Alchirazi indicated no relevant financial relationships.
Michelle Baliss indicated no relevant financial relationships.
Ahmed El Telbany indicated no relevant financial relationships.
Wissam Kiwan indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Speakers Bureau.
Khaled Alsabbagh Alchirazi, MD1, Michelle Baliss, DO2, Ahmed El Telbany, MD, MPH3, Wissam Kiwan, MD4, Mohammad Bilal, MD5. P4094 - Decrease Risk of Intubation and Aspiration Pneumonia Associated with Endoscopic Procedure Among Patients on Glucagon-Like Peptide 1 Receptor Agonists, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
