Tuesday Poster Session
Category: General Endoscopy
P4118 - Performance Metrics of a Novel Single-Use Therapeutic Gastroscope
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

David Hoffman, MSPH, MBA
Ambu USA
Columbia, MD
Presenting Author(s)
Nanlong Liu, MD1, Daniel Marino, MD, MBA2, Christina Cool, MPH3, David Hoffman, MSPH, MBA4
1University of Louisville, Louisville, KY; 2NYU Langone Health, New York, NY; 3Ambu USA, New York, NY; 4Ambu USA, Columbia, MD
Introduction: The FDA cleared the first single-use therapeutic gastroscope (SUTG) (4.2mm working channel) in April 2024. With the SUTG’s larger working channel, compared to most reusable therapeutic gastroscopes (RTGs) (3.7mm), SUTGs provide the added benefit of increased suctioning abilities to help facilitate the evacuation of blood and retained contents from the upper gastrointestinal tract as well as increase safety given sterility and availability benefits. This study aimed to evaluate the performance of the novel SUTG.
Methods: From April to June 2024, 17 physicians at 6 large university hospital systems in the United States completed 30 procedures requiring a therapeutic gastroscope (TG) (4.2mm working channel). After each procedure, physicians completed a survey to assess their experience and the clinical performance of the SUTG rated from 1 (very poor) to 5 (very good). The Ambu aScope Gastro Large was utilized for all cases in the study.
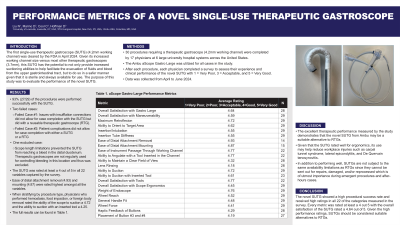
Results: 93% (27/29) of the procedures were performed successfully with the SUTG. Issues with insufflation connections did not allow for procedure completion in one case, while anatomy related complications did not allow for completion of another case with either a SUTG or a RTG – neither case failed due to SUTG performance. Additionally, scope length limitations prevented the SUTG from reaching a bleed in the distal duodenum in one case. This case was excluded from the results because colonoscopes, not TGs, are regularly used for controlling bleeding in this distal anatomy. The SUTG was rated at least a 4 out of 5 in all 22 variables captured by the survey. Ease of distal attachment removal (4.93) and mounting (4.87) were rated highest amongst all variables. When stratified by procedure type, physicians who performed hemostasis, food impaction, or foreign body removal rated the ability of the scope to suction without (4.72) and with an inserted tool (4.25). The overall satisfaction with SUTG’s ergonomics was rated a 4.45. The full results can be found in the table below.
Discussion: The SUTG had a high success rate and was not the cause of failure in any case. The SUTG received high ratings in all 22 categories measured in the survey. Every metric was rated at least a 4 (good) out of 5 (excellent) with the overall satisfaction of the SUTG rating a 4.64 out of 5. The excellent therapeutic performance measured by the study demonstrates that the novel SUTG (Ambu aScope Gastro Large) may be a suitable alternative to RTGs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Nanlong Liu, MD1, Daniel Marino, MD, MBA2, Christina Cool, MPH3, David Hoffman, MSPH, MBA4. P4118 - Performance Metrics of a Novel Single-Use Therapeutic Gastroscope, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Louisville, Louisville, KY; 2NYU Langone Health, New York, NY; 3Ambu USA, New York, NY; 4Ambu USA, Columbia, MD
Introduction: The FDA cleared the first single-use therapeutic gastroscope (SUTG) (4.2mm working channel) in April 2024. With the SUTG’s larger working channel, compared to most reusable therapeutic gastroscopes (RTGs) (3.7mm), SUTGs provide the added benefit of increased suctioning abilities to help facilitate the evacuation of blood and retained contents from the upper gastrointestinal tract as well as increase safety given sterility and availability benefits. This study aimed to evaluate the performance of the novel SUTG.
Methods: From April to June 2024, 17 physicians at 6 large university hospital systems in the United States completed 30 procedures requiring a therapeutic gastroscope (TG) (4.2mm working channel). After each procedure, physicians completed a survey to assess their experience and the clinical performance of the SUTG rated from 1 (very poor) to 5 (very good). The Ambu aScope Gastro Large was utilized for all cases in the study.
Results: 93% (27/29) of the procedures were performed successfully with the SUTG. Issues with insufflation connections did not allow for procedure completion in one case, while anatomy related complications did not allow for completion of another case with either a SUTG or a RTG – neither case failed due to SUTG performance. Additionally, scope length limitations prevented the SUTG from reaching a bleed in the distal duodenum in one case. This case was excluded from the results because colonoscopes, not TGs, are regularly used for controlling bleeding in this distal anatomy. The SUTG was rated at least a 4 out of 5 in all 22 variables captured by the survey. Ease of distal attachment removal (4.93) and mounting (4.87) were rated highest amongst all variables. When stratified by procedure type, physicians who performed hemostasis, food impaction, or foreign body removal rated the ability of the scope to suction without (4.72) and with an inserted tool (4.25). The overall satisfaction with SUTG’s ergonomics was rated a 4.45. The full results can be found in the table below.
Discussion: The SUTG had a high success rate and was not the cause of failure in any case. The SUTG received high ratings in all 22 categories measured in the survey. Every metric was rated at least a 4 (good) out of 5 (excellent) with the overall satisfaction of the SUTG rating a 4.64 out of 5. The excellent therapeutic performance measured by the study demonstrates that the novel SUTG (Ambu aScope Gastro Large) may be a suitable alternative to RTGs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Nanlong Liu indicated no relevant financial relationships.
Daniel Marino indicated no relevant financial relationships.
Christina Cool: Ambu – Employee.
David Hoffman: Ambu USA – Employee.
Nanlong Liu, MD1, Daniel Marino, MD, MBA2, Christina Cool, MPH3, David Hoffman, MSPH, MBA4. P4118 - Performance Metrics of a Novel Single-Use Therapeutic Gastroscope, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
