Tuesday Poster Session
Category: GI Bleeding
P4163 - Is Octreotide Adjunctive Therapy Advantageous in Non-Variceal Gastrointestinal Bleeding? A Systematic Review and Meta-Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
.jpg)
Magnus Chun, MD
Kirk Kerkorian School of Medicine at the University of Nevada
Las Vegas, NV
Presenting Author(s)
Magnus Chun, MD1, Kavita Batra, PhD1, Lily Liu, BS1, Kyaw Min Tun, DO1, Robert G. Gish, MD2
1Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, NV; 2Robert G. Gish Consultants, LLC, San Diego, CA
Introduction: Non-variceal upper gastrointestinal bleeding (NVUGIB) is a common cause of hospitalizations with proton pump inhibitors (PPI) being the mainstay treatment. However, given the high mortality and morbidity of NVUGIB, there is a lack of high-level evidence to show if adjunctive octreotide therapy can improve outcomes. This systematic review and meta-analysis aims to evaluate the outcomes of PPI with octreotide therapy versus PPI monotherapy in treating NVUGIB.
Methods: PubMed, Embase, Web of Science, CINAHL, and Cochrane databases were systematically searched to retrieve English-only, original studies, published between January 1, 2000 to December 31, 2023, which investigated NVUGIB only. Studies investigating variceal bleeding, and those published as reviews, commentary, opinions, and short communications were excluded. Primary outcomes included mortality rate, rebleeding rate, and length of hospital stay between PPI with octreotide therapy versus PPI monotherapy groups. A random-effects model was fit for generating summary estimates. For binary and continuous outcomes, relative risk (RR) and standardized mean differences (SMD) were used as the effect sizes, respectively.
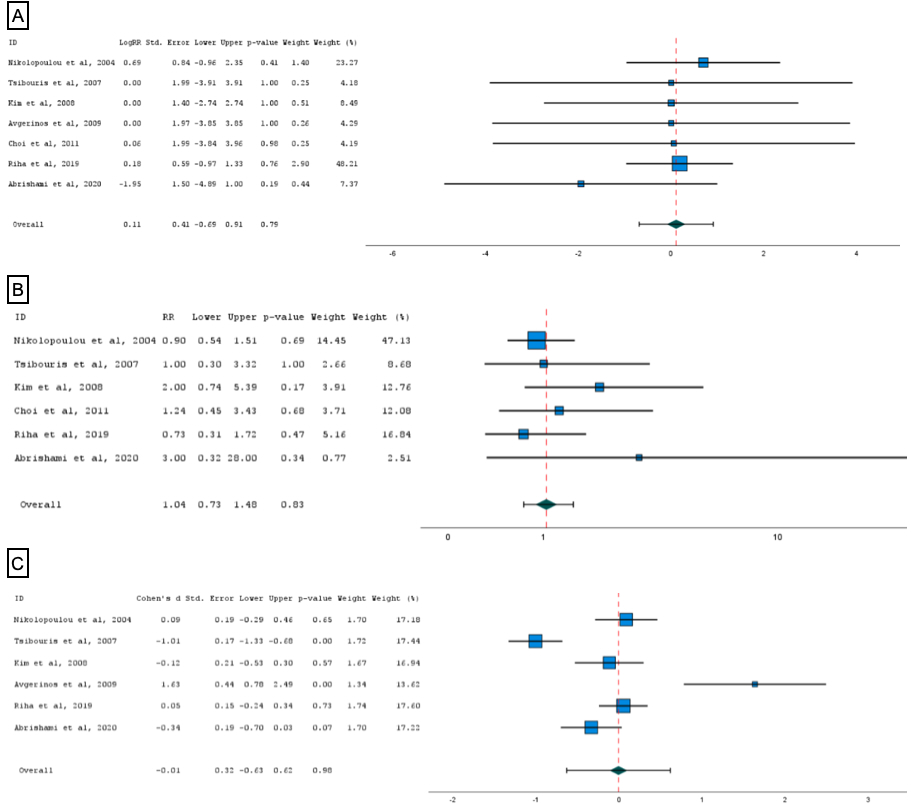
Results: A total of 7 studies with 789 patients had a pooled mortality rate of 2.0% (95%CI: 0%-4.0%) and pooled RR between PPI monotherapy and PPI with octreotide therapy of 0.11 (95%CI: -0.69-0.91, p=0.8). The pooled rebleeding rate was 13% (95%CI: 6%-20%) and pooled RR between groups was 1.04 (95%CI: 0.73,-1.48, p=0.8). The pooled average length of stay in the hospital was 5.27 days (95%CI: 3.72-7.21 days) with insignificant weighted differences between the two groups (-0.01, 95%CI: -0.63, 0.62, p=0.98). No statistically significant differences were noted in surgical management rates (0.00, 95%CI: -0.63-0.32, p=0.99) and amount of blood transfusion (-0.05, 95%CI: -0.32-0.13, p=0.69).
Discussion: Among patients with NVUGIB, octreotide adjunctive therapy had no clinical benefits given no statistically significant differences in the primary outcomes. However, there may be selection bias in who was treated with octreotide that may have influenced these results. Safety signals with octreotide also need to be monitored. Overall, PPI monotherapy is sufficient in patients with NVUGIB.
Disclosures:
Magnus Chun, MD1, Kavita Batra, PhD1, Lily Liu, BS1, Kyaw Min Tun, DO1, Robert G. Gish, MD2. P4163 - Is Octreotide Adjunctive Therapy Advantageous in Non-Variceal Gastrointestinal Bleeding? A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, NV; 2Robert G. Gish Consultants, LLC, San Diego, CA
Introduction: Non-variceal upper gastrointestinal bleeding (NVUGIB) is a common cause of hospitalizations with proton pump inhibitors (PPI) being the mainstay treatment. However, given the high mortality and morbidity of NVUGIB, there is a lack of high-level evidence to show if adjunctive octreotide therapy can improve outcomes. This systematic review and meta-analysis aims to evaluate the outcomes of PPI with octreotide therapy versus PPI monotherapy in treating NVUGIB.
Methods: PubMed, Embase, Web of Science, CINAHL, and Cochrane databases were systematically searched to retrieve English-only, original studies, published between January 1, 2000 to December 31, 2023, which investigated NVUGIB only. Studies investigating variceal bleeding, and those published as reviews, commentary, opinions, and short communications were excluded. Primary outcomes included mortality rate, rebleeding rate, and length of hospital stay between PPI with octreotide therapy versus PPI monotherapy groups. A random-effects model was fit for generating summary estimates. For binary and continuous outcomes, relative risk (RR) and standardized mean differences (SMD) were used as the effect sizes, respectively.
Results: A total of 7 studies with 789 patients had a pooled mortality rate of 2.0% (95%CI: 0%-4.0%) and pooled RR between PPI monotherapy and PPI with octreotide therapy of 0.11 (95%CI: -0.69-0.91, p=0.8). The pooled rebleeding rate was 13% (95%CI: 6%-20%) and pooled RR between groups was 1.04 (95%CI: 0.73,-1.48, p=0.8). The pooled average length of stay in the hospital was 5.27 days (95%CI: 3.72-7.21 days) with insignificant weighted differences between the two groups (-0.01, 95%CI: -0.63, 0.62, p=0.98). No statistically significant differences were noted in surgical management rates (0.00, 95%CI: -0.63-0.32, p=0.99) and amount of blood transfusion (-0.05, 95%CI: -0.32-0.13, p=0.69).
Discussion: Among patients with NVUGIB, octreotide adjunctive therapy had no clinical benefits given no statistically significant differences in the primary outcomes. However, there may be selection bias in who was treated with octreotide that may have influenced these results. Safety signals with octreotide also need to be monitored. Overall, PPI monotherapy is sufficient in patients with NVUGIB.

Figure: (A) Pooled RR for mortality between PPI monotherapy and PPI with octreotide therapy, (B) Pooled RR for rebleeding rate between PPI monotherapy and PPI with octreotide therapy, and (C) Weighted difference for length of hospital stay between PPI monotherapy and PPI with octreotide therapy.
Disclosures:
Magnus Chun indicated no relevant financial relationships.
Kavita Batra indicated no relevant financial relationships.
Lily Liu indicated no relevant financial relationships.
Kyaw Min Tun indicated no relevant financial relationships.
Robert Gish: Abbott – Advisor or Review Panel Member, Consultant. AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker’s contract for promotional talks. Altimmune – Advisor or Review Panel Member, Consultant. AngioCrine – Stock Options. Antios – Advisor or Review Panel Member, Consultant. Arrowhead – Advisor or Review Panel Member, Consultant. BMS – Speaker’s contract for promotional talks. CoCrystal – Minor stock shareholder (liver space noted only). CymaBay Therapeutics – Data safety monitoring board. Durect – Data safety monitoring board. Dynavax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Eiger – Advisor or Review Panel Member, Consultant, Stock Options. Eisai – Advisor or Review Panel Member, Consultant, Speaker’s contract for promotional talks. Enyo – Advisor or Review Panel Member, Consultant. Fujifilm/Wako – Advisory consultant, diagnostic companies. Genentech – Advisor or Review Panel Member, Consultant, Speaker’s contract for promotional talks. Genlantis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Stock Options. Gerson Lehrman Group – Advisor or Review Panel Member, Consultant. Gilead Sciences – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker’s contract for promotional talks. Helios – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. HepaTx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Stock Options. HepQuant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Stock Options. Intercept Pharmaceuticals Inc. – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker’s contract for promotional talks. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Merck – Advisor or Review Panel Member, Consultant. Perspectum – Advisory consultant, diagnostic companies. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Prodigy – Advisory Committee/Board Member, Chair of clinical advisory board. Quest – Advisory consultant, diagnostic companies:. RiboSciences – Minor stock shareholder (liver space noted only). Sonic Incytes – Advisory consultant, diagnostic companies:. Takeda – Data safety monitoring board. Topography Health – Advisor or Review Panel Member, Consultant, Current clinical trials alliance. Venatorx – Advisor or Review Panel Member, Consultant.
Magnus Chun, MD1, Kavita Batra, PhD1, Lily Liu, BS1, Kyaw Min Tun, DO1, Robert G. Gish, MD2. P4163 - Is Octreotide Adjunctive Therapy Advantageous in Non-Variceal Gastrointestinal Bleeding? A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

