Tuesday Poster Session
Category: GI Bleeding
P4231 - Successful Management of Refractory Percutaneous Endoscopic Gastrostomy Site Bleeding Using Hemostatic Peptides
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- MT
Michael Temple, MD
Medical College of Georgia at Augusta University
Augusta, GA
Presenting Author(s)
Michael Temple, MD1, Gomathy A. Nageswaran, MBBS2, Deva Christine T. Tojong, MD1, Loc Ton, MD3, John Erikson Yap, MD, MBA, FACG4
1Medical College of Georgia at Augusta University, Augusta, GA; 2University of Arkansas for Medical Sciences, Little Rock, AR; 3Kaiser Permanente, South Sacramento, CA; 4Metrodora Institute, West Valley City, UT
Introduction: Percutaneous endoscopic gastrostomy (PEG) tube placement is a widely performed endoscopic procedure, providing an alternative enteral feeding route for patients. While generally safe and straightforward, it can occasionally lead to intra-operative and post-operative bleeding. Novel hemostatic agents have emerged as effective rescue therapies when conventional methods fail, such as using self-assembling hemostatic peptide hydrogels to seal bleeding sites.
Case Description/Methods: A 66-year-old male was admitted for a cerebrovascular accident (CVA) due to atrial fibrillation. He exhibited dysphagia and was recommended for alternate means of nutrition. A PEG was performed after holding anticoagulation (AC) beforehand and for 48 hours post-procedure. Upon resumption of AC, the patient developed melena and experienced a drop in hemoglobin, necessitating transfusion of two units of packed red blood cells. A subsequent endoscopy identified bleeding from the PEG site. The PEG bumper was tightened for 24 hours to facilitate hemostasis, and AC was temporarily suspended. AC was resumed five days later after hemoglobin levels stabilized, but bleeding recurred, requiring further transfusion. A repeat endoscopy revealed persistent oozing from the PEG site, prompting endoscopic application of hemostatic peptide (PuraStat®) to the gastric and abdominal sides of the PEG site fistula. The patient remained stable with no further bleeding. AC was successfully resumed, and the patient was safely discharged.
Discussion: Despite its generally favorable safety profile, percutaneous endoscopic gastrostomy (PEG) placement carries inherent risks of complications such as tube dislodgement, site infection, minor oozing, and, in rare cases, severe bleeding. Contributing factors may include procedural technique, underlying bleeding disorders, or recent anticoagulation therapy, as observed in this case. Hemostatic peptides stand out among available hemostatic agents for its transparency, which facilitates optimal visualization during hemostasis. In this instance, we successfully employed this hemostatic peptide hydrogel to effectively manage recurrent bleeding at the PEG insertion site, resolving the issue where conventional hemostatic measures had proven inadequate, and without encountering adverse effects. While these initial outcomes are promising, further rigorous studies are needed to establish its efficacy and safety profile, particularly in patients requiring concurrent anticoagulation therapy.
Disclosures:
Michael Temple, MD1, Gomathy A. Nageswaran, MBBS2, Deva Christine T. Tojong, MD1, Loc Ton, MD3, John Erikson Yap, MD, MBA, FACG4. P4231 - Successful Management of Refractory Percutaneous Endoscopic Gastrostomy Site Bleeding Using Hemostatic Peptides, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Medical College of Georgia at Augusta University, Augusta, GA; 2University of Arkansas for Medical Sciences, Little Rock, AR; 3Kaiser Permanente, South Sacramento, CA; 4Metrodora Institute, West Valley City, UT
Introduction: Percutaneous endoscopic gastrostomy (PEG) tube placement is a widely performed endoscopic procedure, providing an alternative enteral feeding route for patients. While generally safe and straightforward, it can occasionally lead to intra-operative and post-operative bleeding. Novel hemostatic agents have emerged as effective rescue therapies when conventional methods fail, such as using self-assembling hemostatic peptide hydrogels to seal bleeding sites.
Case Description/Methods: A 66-year-old male was admitted for a cerebrovascular accident (CVA) due to atrial fibrillation. He exhibited dysphagia and was recommended for alternate means of nutrition. A PEG was performed after holding anticoagulation (AC) beforehand and for 48 hours post-procedure. Upon resumption of AC, the patient developed melena and experienced a drop in hemoglobin, necessitating transfusion of two units of packed red blood cells. A subsequent endoscopy identified bleeding from the PEG site. The PEG bumper was tightened for 24 hours to facilitate hemostasis, and AC was temporarily suspended. AC was resumed five days later after hemoglobin levels stabilized, but bleeding recurred, requiring further transfusion. A repeat endoscopy revealed persistent oozing from the PEG site, prompting endoscopic application of hemostatic peptide (PuraStat®) to the gastric and abdominal sides of the PEG site fistula. The patient remained stable with no further bleeding. AC was successfully resumed, and the patient was safely discharged.
Discussion: Despite its generally favorable safety profile, percutaneous endoscopic gastrostomy (PEG) placement carries inherent risks of complications such as tube dislodgement, site infection, minor oozing, and, in rare cases, severe bleeding. Contributing factors may include procedural technique, underlying bleeding disorders, or recent anticoagulation therapy, as observed in this case. Hemostatic peptides stand out among available hemostatic agents for its transparency, which facilitates optimal visualization during hemostasis. In this instance, we successfully employed this hemostatic peptide hydrogel to effectively manage recurrent bleeding at the PEG insertion site, resolving the issue where conventional hemostatic measures had proven inadequate, and without encountering adverse effects. While these initial outcomes are promising, further rigorous studies are needed to establish its efficacy and safety profile, particularly in patients requiring concurrent anticoagulation therapy.

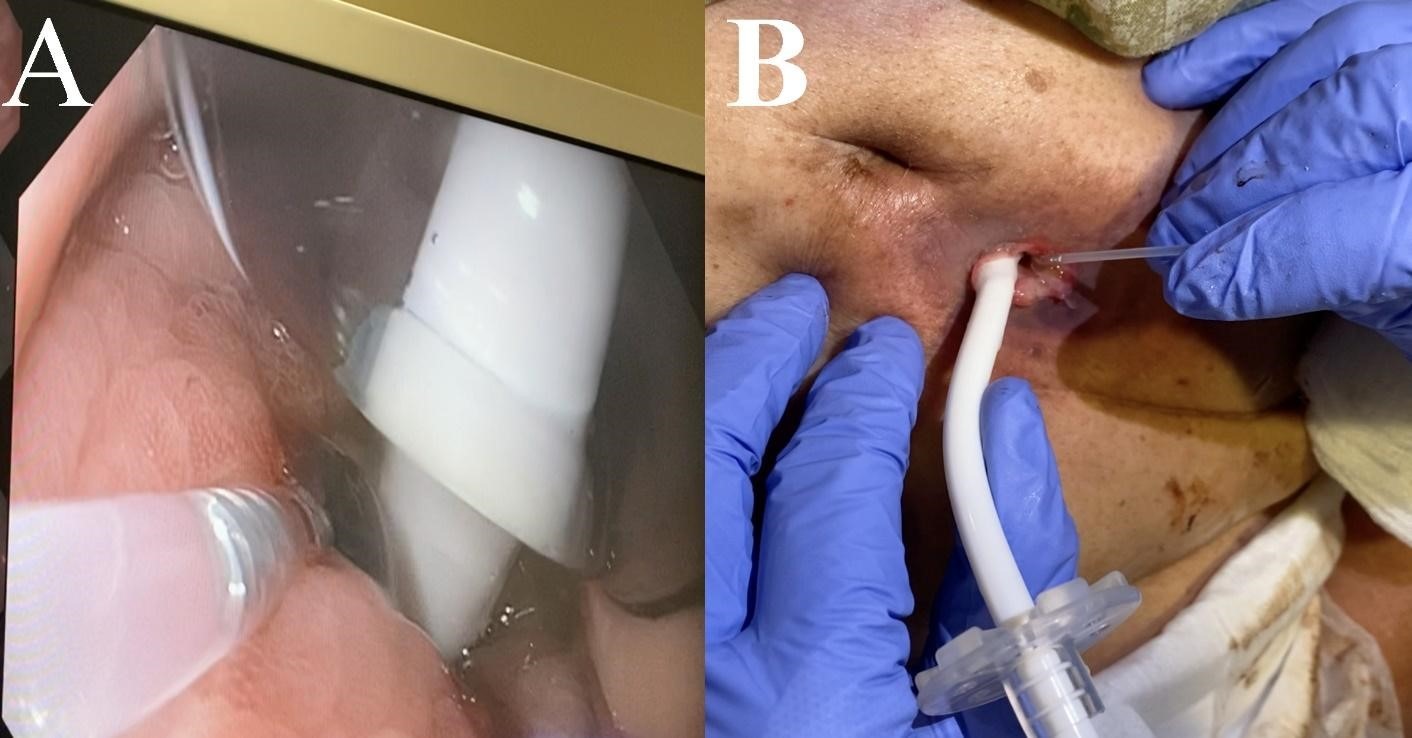
Figure: Figure 1A. Endoscopic picture showing application of Purastat to the gastric mucosa side on the PEG site fistula.
Figure 1B. Application of Purastat to the abdominal side of PEG site fistula.
Figure 1B. Application of Purastat to the abdominal side of PEG site fistula.
Disclosures:
Michael Temple indicated no relevant financial relationships.
Gomathy Nageswaran indicated no relevant financial relationships.
Deva Christine Tojong indicated no relevant financial relationships.
Loc Ton indicated no relevant financial relationships.
John Erikson Yap indicated no relevant financial relationships.
Michael Temple, MD1, Gomathy A. Nageswaran, MBBS2, Deva Christine T. Tojong, MD1, Loc Ton, MD3, John Erikson Yap, MD, MBA, FACG4. P4231 - Successful Management of Refractory Percutaneous Endoscopic Gastrostomy Site Bleeding Using Hemostatic Peptides, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
