Tuesday Poster Session
Category: Functional Bowel Disease
P4069 - Crofelemer Improves Symptoms of Chronic Idiopathic Diarrhea
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Brooks D. Cash, MD, FACG
The University of Texas Health Science Center at Houston
Houston, TX
Presenting Author(s)
Brooks D. Cash, MD, FACG1, Ryan Butcher, MS2, Beena Sattar, MD1, Iram Abassi, MD1
1McGovern Medical School at UTHealth, Houston, TX; 2Charleston Area Medical Center, Madison, WV
Introduction: Despite significant advances in diagnostics, no organic etiology can be determined in a sizable proportion of patients suffering from chronic diarrhea. Variable success for this condition has been observed with non-pharmacologic measures, over the counter therapies, and prescription agents. We conducted a pilot study of patients with idiopathic chronic diarrhea to assess their response to crofelemer, a Food and Drug Administration-approved therapy for HIV-associated diarrhea.
Methods: We prospectively recruited patients 18-75 years of age with chronic idiopathic diarrhea defined as 3 non-bloody loose stools per day or more than 20 non-bloody loose stools per week for more ≥ 4 weeks and a Bristol stool form scale (BSFS) of 6/7 with >50% stools. Eligible patients had a negative extensive, guideline-driven evaluation including stool, blood, breath tests, and bi-directional endoscopy with biopsy. Patients were offered a 28-day open-label course of crofelemer. Primary response was defined as a 50% decrease in mean BSFS 6/7 stool count per week by the end of week 4 and secondary response was defined as a decrease in average stool consistency by more than 2 levels in the BSFS from baseline to the end of treatment. Improvement with crofelemer was defined as meeting primary and/or secondary response criteria.
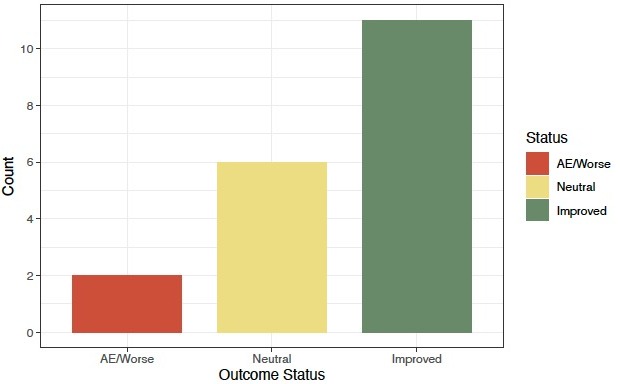
Results: Outcomes were available for 19 patients with chronic idiopathic diarrhea who completed a 28-day course of crofelemer (74% female; mean age 57.2 years). Two patients (10.5%) described worsening diarrhea, 6 (31.6%) described a neutral effect, and 11 (57.9%) improved with crofelemer (Figure 1). Using a binary outcome, 58.8% (11/19) improved with crofelemer while 42.1% (8/19) did not improve.
Discussion: Crofelemer, the first and only botanical drug approved by the Food and Drug Administration, is derived from the red latex of the <em>Croton lechleri</em> tree. It is an acid-labile proanthrocyanidin oligomer that works through dual inhibition of the cAMP-stimulated cystic fibrosis transmembrane regulator and calcium-activated chloride channels at the luminal membrane of enterocytes to normalize the flow of chloride and water into the gastrointestinal tract, leading to anti-secretory effects. In the pilot study we found that crofelemer improved the symptoms of nearly 60% of patients with idiopathic chronic diarrhea. Crofelemer has minimal systemic absorption and no known active metabolites and was well tolerated in our study. These results should prompt a larger evaluation of the effects of crofelemer in patients with chronic idiopathic diarrhea.
Disclosures:
Brooks D. Cash, MD, FACG1, Ryan Butcher, MS2, Beena Sattar, MD1, Iram Abassi, MD1. P4069 - Crofelemer Improves Symptoms of Chronic Idiopathic Diarrhea, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1McGovern Medical School at UTHealth, Houston, TX; 2Charleston Area Medical Center, Madison, WV
Introduction: Despite significant advances in diagnostics, no organic etiology can be determined in a sizable proportion of patients suffering from chronic diarrhea. Variable success for this condition has been observed with non-pharmacologic measures, over the counter therapies, and prescription agents. We conducted a pilot study of patients with idiopathic chronic diarrhea to assess their response to crofelemer, a Food and Drug Administration-approved therapy for HIV-associated diarrhea.
Methods: We prospectively recruited patients 18-75 years of age with chronic idiopathic diarrhea defined as 3 non-bloody loose stools per day or more than 20 non-bloody loose stools per week for more ≥ 4 weeks and a Bristol stool form scale (BSFS) of 6/7 with >50% stools. Eligible patients had a negative extensive, guideline-driven evaluation including stool, blood, breath tests, and bi-directional endoscopy with biopsy. Patients were offered a 28-day open-label course of crofelemer. Primary response was defined as a 50% decrease in mean BSFS 6/7 stool count per week by the end of week 4 and secondary response was defined as a decrease in average stool consistency by more than 2 levels in the BSFS from baseline to the end of treatment. Improvement with crofelemer was defined as meeting primary and/or secondary response criteria.
Results: Outcomes were available for 19 patients with chronic idiopathic diarrhea who completed a 28-day course of crofelemer (74% female; mean age 57.2 years). Two patients (10.5%) described worsening diarrhea, 6 (31.6%) described a neutral effect, and 11 (57.9%) improved with crofelemer (Figure 1). Using a binary outcome, 58.8% (11/19) improved with crofelemer while 42.1% (8/19) did not improve.
Discussion: Crofelemer, the first and only botanical drug approved by the Food and Drug Administration, is derived from the red latex of the <em>Croton lechleri</em> tree. It is an acid-labile proanthrocyanidin oligomer that works through dual inhibition of the cAMP-stimulated cystic fibrosis transmembrane regulator and calcium-activated chloride channels at the luminal membrane of enterocytes to normalize the flow of chloride and water into the gastrointestinal tract, leading to anti-secretory effects. In the pilot study we found that crofelemer improved the symptoms of nearly 60% of patients with idiopathic chronic diarrhea. Crofelemer has minimal systemic absorption and no known active metabolites and was well tolerated in our study. These results should prompt a larger evaluation of the effects of crofelemer in patients with chronic idiopathic diarrhea.

Figure: Response to Crofelemer in Patients with Chronic Idiopathic Diarrhea (n=19)
Disclosures:
Brooks Cash: Abbvie – Consultant, Speakers Bureau. Alnylam – Speakers Bureau. Ardelyx – Consultant, Speakers Bureau. Astra Zeneca – Consultant, Speakers Bureau. Phathom – Consultant, Speakers Bureau. QOL – Speakers Bureau. Salix – Speakers Bureau. Vibrant Advisory Board – Advisory Committee/Board Member.
Ryan Butcher indicated no relevant financial relationships.
Beena Sattar indicated no relevant financial relationships.
Iram Abassi indicated no relevant financial relationships.
Brooks D. Cash, MD, FACG1, Ryan Butcher, MS2, Beena Sattar, MD1, Iram Abassi, MD1. P4069 - Crofelemer Improves Symptoms of Chronic Idiopathic Diarrhea, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

