Monday Poster Session
Category: Liver
P3067 - From Sweet to Sour: A Case of Papaya Leaf Extract Induced Hepatotoxicity
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Alexander J. Dile, DO
University of Cincinnati Medical Center
Cincinnati, OH
Presenting Author(s)
Alexander J. Dile, DO1, Patrick Carey, MD1, Divya Sharma, MD1, Yeshika Sharma, MD2, Michael Schoech, MD1
1University of Cincinnati Medical Center, Cincinnati, OH; 2University of Cincinnati, Cincinnati, OH
Introduction: The widespread availability of dietary supplements, coupled with limited regulations and testing, often implicates them in cases of liver injury. Papaya leaf extract is marketed as a safe supplement with anti-inflammatory properties and benefits against thrombocytopenia. While there have been no prior documented cases of associated liver injuries in humans, we are presenting a unique instance of such an occurrence causing a cholestatic pattern of liver injury.
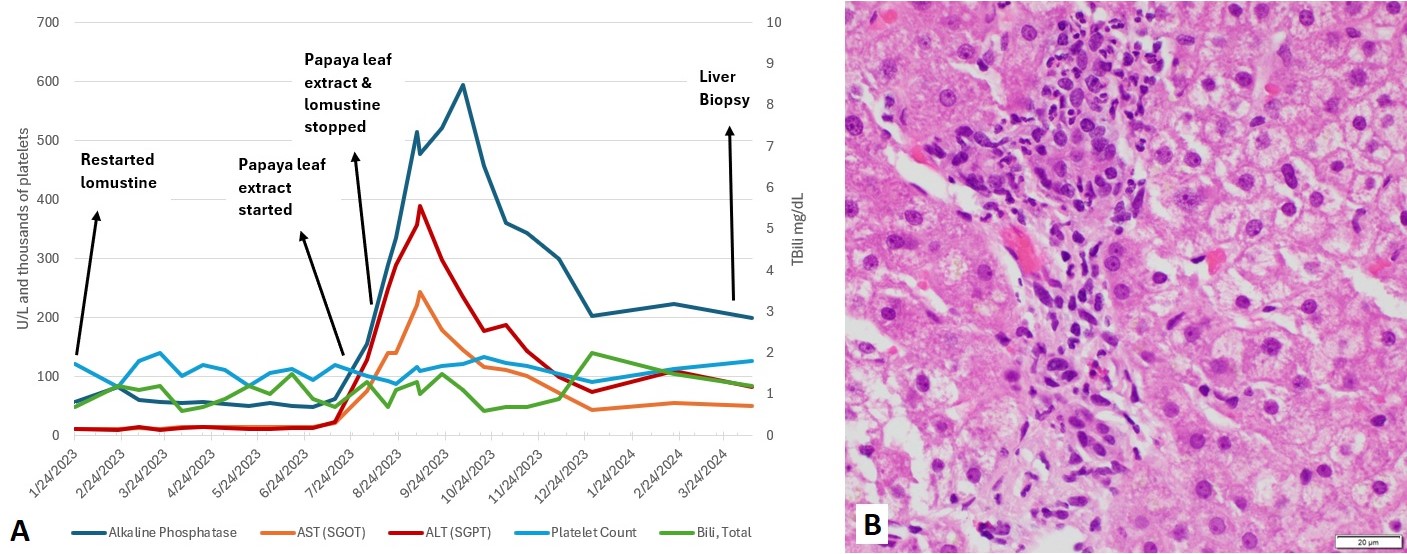
Case Description/Methods: A 43-year-old male with history of a grade III astrocytoma diagnosed in 2017 stable on bevacizumab and lomustine was found to have an acute elevation in his liver enzymes: ALP 156, AST 76, ALT 128, T Bili 1.3. He endorsed taking 1 tablespoon daily of papaya leaf extract over the prior 2 weeks for its supposed benefits to platelet production. Given the concern for drug induced liver injury, it was discontinued. Despite cessation, his liver enzymes continued to rise to a peak of ALP 594, AST 243, ALT 389 (Figure 1). Bevacizumab infusions were initially held but then restarted while lomustine was held indefinitely. CT abdomen and MRCP were unremarkable. Less common viral causes like HSV, VZV, CMV, EBV, ANA, AMA, anti-F actin, IgG level, and iron studies were unrevealing. He underwent liver biopsy which showed non-specific biliary ductal damage reflective of medication induced injury (Figure 2). At time of biopsy, the patient's liver enzymes had down trended without further intervention. He abstained from further use of papaya leaf extract.
Discussion: Although no human studies have been conducted, several animal studies have suggested a potential correlation between transient aminotransferase elevations and papaya extract administration. Bevacizumab is not known to cause any aminotransferase elevations. While lomustine has potential for a cholestatic pattern of injury typically 3-4 months into therapy, our patient had been on it for over 8 months and in previous years without any liver abnormalities. So while certain chemotherapy agents are known to be hepatotoxic, they may not always be the sole culprit. Thus, accurate medication reconciliation, including over-the-counter supplements, is vital in elucidating the cause of liver injuries, especially when patients are concurrently using other hepatotoxic agents. The unregulated nature of supplements introduces a risk of unpredictable reactions, underscoring the importance of physicians being fully informed on all medications and supplements their patients consume.
Disclosures:
Alexander J. Dile, DO1, Patrick Carey, MD1, Divya Sharma, MD1, Yeshika Sharma, MD2, Michael Schoech, MD1. P3067 - From Sweet to Sour: A Case of Papaya Leaf Extract Induced Hepatotoxicity, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Cincinnati Medical Center, Cincinnati, OH; 2University of Cincinnati, Cincinnati, OH
Introduction: The widespread availability of dietary supplements, coupled with limited regulations and testing, often implicates them in cases of liver injury. Papaya leaf extract is marketed as a safe supplement with anti-inflammatory properties and benefits against thrombocytopenia. While there have been no prior documented cases of associated liver injuries in humans, we are presenting a unique instance of such an occurrence causing a cholestatic pattern of liver injury.
Case Description/Methods: A 43-year-old male with history of a grade III astrocytoma diagnosed in 2017 stable on bevacizumab and lomustine was found to have an acute elevation in his liver enzymes: ALP 156, AST 76, ALT 128, T Bili 1.3. He endorsed taking 1 tablespoon daily of papaya leaf extract over the prior 2 weeks for its supposed benefits to platelet production. Given the concern for drug induced liver injury, it was discontinued. Despite cessation, his liver enzymes continued to rise to a peak of ALP 594, AST 243, ALT 389 (Figure 1). Bevacizumab infusions were initially held but then restarted while lomustine was held indefinitely. CT abdomen and MRCP were unremarkable. Less common viral causes like HSV, VZV, CMV, EBV, ANA, AMA, anti-F actin, IgG level, and iron studies were unrevealing. He underwent liver biopsy which showed non-specific biliary ductal damage reflective of medication induced injury (Figure 2). At time of biopsy, the patient's liver enzymes had down trended without further intervention. He abstained from further use of papaya leaf extract.
Discussion: Although no human studies have been conducted, several animal studies have suggested a potential correlation between transient aminotransferase elevations and papaya extract administration. Bevacizumab is not known to cause any aminotransferase elevations. While lomustine has potential for a cholestatic pattern of injury typically 3-4 months into therapy, our patient had been on it for over 8 months and in previous years without any liver abnormalities. So while certain chemotherapy agents are known to be hepatotoxic, they may not always be the sole culprit. Thus, accurate medication reconciliation, including over-the-counter supplements, is vital in elucidating the cause of liver injuries, especially when patients are concurrently using other hepatotoxic agents. The unregulated nature of supplements introduces a risk of unpredictable reactions, underscoring the importance of physicians being fully informed on all medications and supplements their patients consume.

Figure: (A) Aminotransferases, bilirubin and platelet levels graphed over time with corresponding start and stop dates of papaya leaf extract ingestion. (B) Liver biopsy with hematoxylin and eosin stain showing neutrophil predominance and bile duct damage
Disclosures:
Alexander Dile indicated no relevant financial relationships.
Patrick Carey indicated no relevant financial relationships.
Divya Sharma indicated no relevant financial relationships.
Yeshika Sharma: Intercept Pharmaceuticals – Speakers Bureau.
Michael Schoech indicated no relevant financial relationships.
Alexander J. Dile, DO1, Patrick Carey, MD1, Divya Sharma, MD1, Yeshika Sharma, MD2, Michael Schoech, MD1. P3067 - From Sweet to Sour: A Case of Papaya Leaf Extract Induced Hepatotoxicity, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
