Tuesday Poster Session
Category: Biliary/Pancreas
P3469 - What’s Too Much to Ask of EUS-Guided Chemoablation? A Case Series Review of High Oncologic Risk EUS-Guided Pancreatic Cyst Chemoablation: Efficacy, Safety, and Durability
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Allison R. Leisgang, DO
Penn State Health
Hershey, PA
Presenting Author(s)
Allison R. Leisgang, DO1, John M. DeWitt, MD, FACG2, Charles Vining, MD1, Charles E. Dye, MD1, Sydney Rhoades, BS1, Rushin Brahmbhatt, MD1, Matthew T. Moyer, MD1
1Penn State Health, Hershey, PA; 2Indiana University School of Medicine, Indianapolis, IN
Introduction: The most common pancreatic cysts are neoplastic and include mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN) which carry the potential for malignant transformation over time. Endoscopic ultrasound (EUS)-guided pancreatic cyst chemoablation has emerged as an innovative and promising minimally invasive approach for appropriately selected pancreatic cysts; however, certain high-risk oncologic features have generally been relative contraindications. When patients with these high-risk oncologic features are not surgical candidates, then an expanded treatment criteria may be appropriate to allow treatment of these high-risk cysts by EUS-guided chemoablation on a selective basis. Here, we evaluate the efficacy and safety of EUS-guided chemoablation for mucinous type pancreatic cysts with high-risk oncologic features which precluded them from participation in current prospective chemoablation clinical trials and are not surgical candidates.
Methods: 19 patients with the following high-risk features were retrospectively evaluated: thick walls/septations >3 mm, mural nodule of > 5 mm or solid component, elevated CA 19-9, main duct dilation (5-9 mm), pancreatic duct stricture with tail atrophy, as well as technical relative contraindications of size > 5 cm or >4 septations. Patients underwent 1-5 sessions of alcohol-free, EUS-guided, pancreatic cyst chemoablation as previously described. Surveillance was performed by enhanced MRI-MRCP at 6-month intervals.
Results: At 1 year, 84% of patients had a greater than 75% reduction in volume (partial or complete resolution) with 42% having >95% reduction (complete resolution) of their pancreatic cystic tumor. There were two (3%) serious adverse events within 30 days of the chemoablation procedures and no patient developed adenocarcinoma in a treated cyst over a mean of 3 years of follow-up with radiographic post-treatment surveillance.
Discussion: EUS-guided pancreatic cyst chemoablation is emerging as a minimally invasive treatment for appropriately selected mucinous cysts using previously described selection criteria. These current results suggest that in an expert, multidisciplinary setting, EUS-guided chemoablation can be considered as an effective treatment alternative in higher risk “expanded oncologic criteria” cysts when surgical risks are otherwise prohibitive.
Disclosures:
Allison R. Leisgang, DO1, John M. DeWitt, MD, FACG2, Charles Vining, MD1, Charles E. Dye, MD1, Sydney Rhoades, BS1, Rushin Brahmbhatt, MD1, Matthew T. Moyer, MD1. P3469 - What’s Too Much to Ask of EUS-Guided Chemoablation? A Case Series Review of High Oncologic Risk EUS-Guided Pancreatic Cyst Chemoablation: Efficacy, Safety, and Durability, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Penn State Health, Hershey, PA; 2Indiana University School of Medicine, Indianapolis, IN
Introduction: The most common pancreatic cysts are neoplastic and include mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN) which carry the potential for malignant transformation over time. Endoscopic ultrasound (EUS)-guided pancreatic cyst chemoablation has emerged as an innovative and promising minimally invasive approach for appropriately selected pancreatic cysts; however, certain high-risk oncologic features have generally been relative contraindications. When patients with these high-risk oncologic features are not surgical candidates, then an expanded treatment criteria may be appropriate to allow treatment of these high-risk cysts by EUS-guided chemoablation on a selective basis. Here, we evaluate the efficacy and safety of EUS-guided chemoablation for mucinous type pancreatic cysts with high-risk oncologic features which precluded them from participation in current prospective chemoablation clinical trials and are not surgical candidates.
Methods: 19 patients with the following high-risk features were retrospectively evaluated: thick walls/septations >3 mm, mural nodule of > 5 mm or solid component, elevated CA 19-9, main duct dilation (5-9 mm), pancreatic duct stricture with tail atrophy, as well as technical relative contraindications of size > 5 cm or >4 septations. Patients underwent 1-5 sessions of alcohol-free, EUS-guided, pancreatic cyst chemoablation as previously described. Surveillance was performed by enhanced MRI-MRCP at 6-month intervals.
Results: At 1 year, 84% of patients had a greater than 75% reduction in volume (partial or complete resolution) with 42% having >95% reduction (complete resolution) of their pancreatic cystic tumor. There were two (3%) serious adverse events within 30 days of the chemoablation procedures and no patient developed adenocarcinoma in a treated cyst over a mean of 3 years of follow-up with radiographic post-treatment surveillance.
Discussion: EUS-guided pancreatic cyst chemoablation is emerging as a minimally invasive treatment for appropriately selected mucinous cysts using previously described selection criteria. These current results suggest that in an expert, multidisciplinary setting, EUS-guided chemoablation can be considered as an effective treatment alternative in higher risk “expanded oncologic criteria” cysts when surgical risks are otherwise prohibitive.

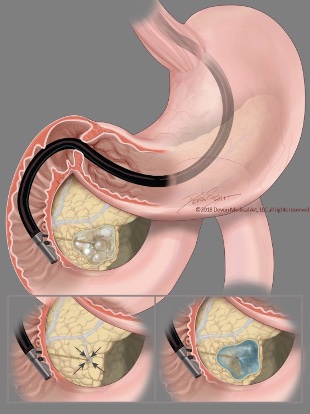
Figure: Figure 1. EUS-guided pancreatic cyst chemoablation technique. First, the needle is guided to the center of the mucinous cyst and all fluid is removed leaving a small rim of fluid around the needle tip. The same volume of the chemoablation admixture is then infused, reconstituting the cyst to its original size and dimensions.
Disclosures:
Allison R. Leisgang indicated no relevant financial relationships.
John DeWitt indicated no relevant financial relationships.
Charles Vining indicated no relevant financial relationships.
Charles E. Dye indicated no relevant financial relationships.
Sydney Rhoades indicated no relevant financial relationships.
Rushin Brahmbhatt indicated no relevant financial relationships.
Matthew T. Moyer indicated no relevant financial relationships.
Allison R. Leisgang, DO1, John M. DeWitt, MD, FACG2, Charles Vining, MD1, Charles E. Dye, MD1, Sydney Rhoades, BS1, Rushin Brahmbhatt, MD1, Matthew T. Moyer, MD1. P3469 - What’s Too Much to Ask of EUS-Guided Chemoablation? A Case Series Review of High Oncologic Risk EUS-Guided Pancreatic Cyst Chemoablation: Efficacy, Safety, and Durability, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

