Tuesday Poster Session
Category: Esophagus
P3943 - Evaluation of Adverse Events of Dupixent When Used for Eosinophilic Esophagitis- A Pharmacovigilance Study From FAERS Database
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Keerthi Mannumbeth Renjith, MD
Rochester Regional Health, Unity Hospital
Greece, NY
Presenting Author(s)
Tausif Syed, MD1, Keerthi Mannumbeth Renjith, MD2, Prajjwol Bhatta, MD3, Sheen Kamal, MD4, Farooq Chaudhary, MD2, Asim Mushtaq, MD3
1Rochester General Hospital, Greece, NY; 2Rochester Regional Health, Unity Hospital, Greece, NY; 3Rochester General Hospital, Rochester, NY; 4Rochester Regional Health, Rochester, NY
Introduction: Eosinophilic esophagitis (EoE) is one of the common disorders encountered by gastroenterologist. It has previously been treated with proton pump inhibitors and steroids. Dupixent is a medication that was approved by FDA in 2022 for eosinophilic esophagitis. Dupixent is an IL-4 and IL-13 inhibitor. There have been no guidelines currently when to use Dupixent, but it has been used in resistant cases that do not respond to PPI therapy. The majority of gastroenterologist are hesitant to use steroid for long term and as a result they prefer to use Dupixent in such cases. The objective was to study the side effects of the medication Dupixent with the help of real-world data.
Methods: We conducted retrospective data analysis of all the serious cases of side effects with the use of Dupixent in EOE. The data was collected from FDA adverse event reporting system (FAERS) The data was restricted to 2022 when the FDA approved the use of this medication for EOE.
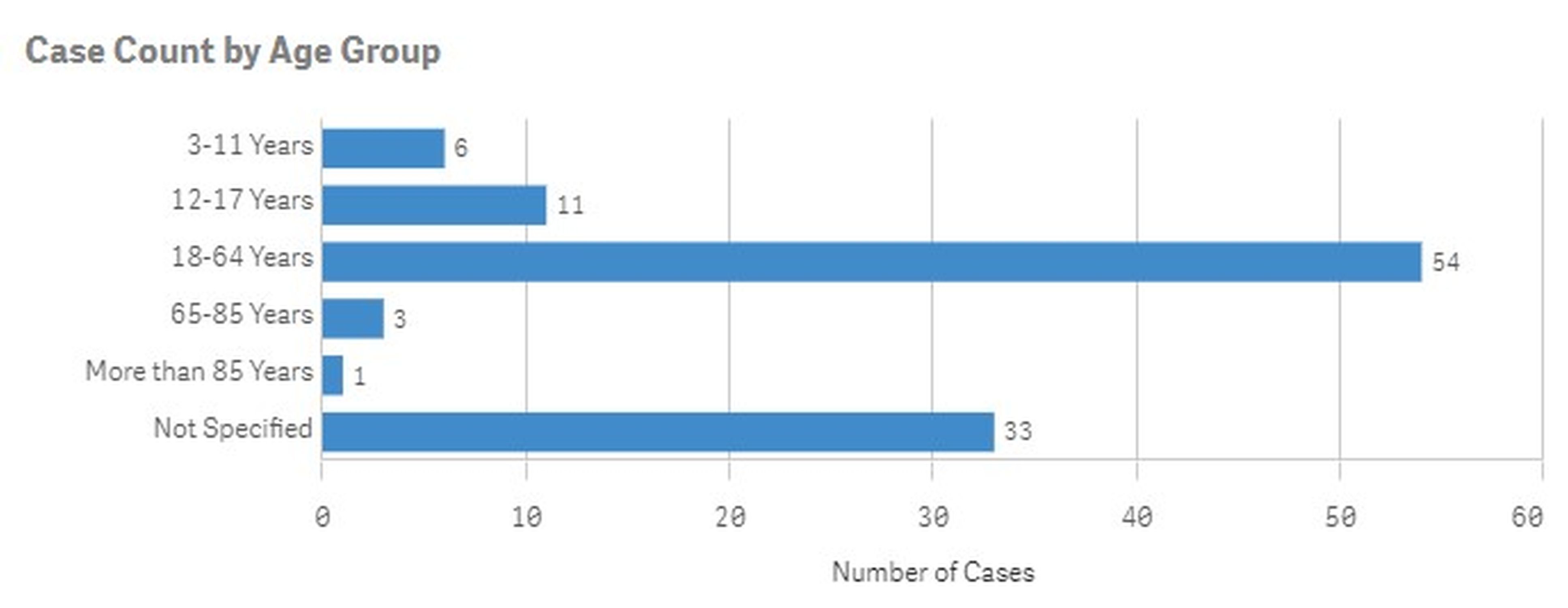
Results: There were a total of 4448 adverse events noted with the use of Dupixent in EoE in FAERS data base from 2022 to March 31, 2024. Of 4448 cases, there were 324 (7.3%) cases which were labelled as serious including deaths. The most common serious side effects were injection site pain (13.85%), injection site erythema (8.97%) and injection site swelling (8.5%). Of the 324 serious cases, inappropriate use was 10.8% cases. The other common serious complications were dysphagia, arthralgia, anaphylactic reaction, etc. There were total 108 cases (2.4%) which required hospitalization. The maximum number of patients hospitalized were in the age group 18-64 ( Figure 1). There were total six deaths which were linked to the use of Dupixent in EOE which included RTA, suicide (X2), PE, ALS and anaphylactoid reaction.
Discussion: Dupixent appears to be a safe medication with the local injection site complications making the bulk of the reported serious adverse events. But gastroenterologist should be aware that around 2.4 % adverse event cases needed hospitalization and they should discuss with the patient about these complications before starting this medication.
Disclosures:
Tausif Syed, MD1, Keerthi Mannumbeth Renjith, MD2, Prajjwol Bhatta, MD3, Sheen Kamal, MD4, Farooq Chaudhary, MD2, Asim Mushtaq, MD3. P3943 - Evaluation of Adverse Events of Dupixent When Used for Eosinophilic Esophagitis- A Pharmacovigilance Study From FAERS Database, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Rochester General Hospital, Greece, NY; 2Rochester Regional Health, Unity Hospital, Greece, NY; 3Rochester General Hospital, Rochester, NY; 4Rochester Regional Health, Rochester, NY
Introduction: Eosinophilic esophagitis (EoE) is one of the common disorders encountered by gastroenterologist. It has previously been treated with proton pump inhibitors and steroids. Dupixent is a medication that was approved by FDA in 2022 for eosinophilic esophagitis. Dupixent is an IL-4 and IL-13 inhibitor. There have been no guidelines currently when to use Dupixent, but it has been used in resistant cases that do not respond to PPI therapy. The majority of gastroenterologist are hesitant to use steroid for long term and as a result they prefer to use Dupixent in such cases. The objective was to study the side effects of the medication Dupixent with the help of real-world data.
Methods: We conducted retrospective data analysis of all the serious cases of side effects with the use of Dupixent in EOE. The data was collected from FDA adverse event reporting system (FAERS) The data was restricted to 2022 when the FDA approved the use of this medication for EOE.
Results: There were a total of 4448 adverse events noted with the use of Dupixent in EoE in FAERS data base from 2022 to March 31, 2024. Of 4448 cases, there were 324 (7.3%) cases which were labelled as serious including deaths. The most common serious side effects were injection site pain (13.85%), injection site erythema (8.97%) and injection site swelling (8.5%). Of the 324 serious cases, inappropriate use was 10.8% cases. The other common serious complications were dysphagia, arthralgia, anaphylactic reaction, etc. There were total 108 cases (2.4%) which required hospitalization. The maximum number of patients hospitalized were in the age group 18-64 ( Figure 1). There were total six deaths which were linked to the use of Dupixent in EOE which included RTA, suicide (X2), PE, ALS and anaphylactoid reaction.
Discussion: Dupixent appears to be a safe medication with the local injection site complications making the bulk of the reported serious adverse events. But gastroenterologist should be aware that around 2.4 % adverse event cases needed hospitalization and they should discuss with the patient about these complications before starting this medication.

Figure: figure 1- hospitalization due to adverse events
Disclosures:
Tausif Syed indicated no relevant financial relationships.
Keerthi Mannumbeth Renjith indicated no relevant financial relationships.
Prajjwol Bhatta indicated no relevant financial relationships.
Sheen Kamal indicated no relevant financial relationships.
Farooq Chaudhary indicated no relevant financial relationships.
Asim Mushtaq indicated no relevant financial relationships.
Tausif Syed, MD1, Keerthi Mannumbeth Renjith, MD2, Prajjwol Bhatta, MD3, Sheen Kamal, MD4, Farooq Chaudhary, MD2, Asim Mushtaq, MD3. P3943 - Evaluation of Adverse Events of Dupixent When Used for Eosinophilic Esophagitis- A Pharmacovigilance Study From FAERS Database, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
