Tuesday Poster Session
Category: Colon
P3778 - Obinutuzumab: A Rare Cause of Colitis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- SS
Sean Sullivan, DO
Bryn Mawr
Norristown, PA
Presenting Author(s)
Sean Sullivan, DO1, Julie Sullivan, DO2, Steven Stanek, MD3
1Bryn Mawr, Norristown, PA; 2Einstein Medical Center, Norristown, PA; 3Einstein Healthcare Network, East Norriton, PA
Introduction: Colitis is an increasingly recognized adverse effect of many chemotherapeutic agents. Cytotoxic agents and checkpoint inhibitor immunotherapies have been well documented to cause colitis, leading to an increasing number of hospital admissions and increased morbidity. Here we present a case of colitis secondary to Obinutuzumab, a CD 20 monoclonal antibody used to treat B-cell mediated malignancies, a side effect rarely reported in the literature.
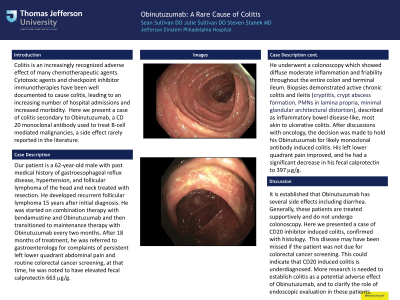
Case Description/Methods: Our patient is a 62-year-old male with past medical history of gastroesophageal reflux disease, hypertension, and follicular lymphoma of the head and neck treated with resection. He developed recurrent follicular lymphoma 15 years after initial diagnosis. He was started on combination therapy with bendamustine and Obinutuzumab and then transitioned to maintenance therapy with Obinutuzumab every two months. After 18 months of treatment, he was referred to gastroenterology for complaints of persistent left lower quadrant abdominal pain and routine colorectal cancer screening, at that time, he was noted to have elevated fecal calprotectin 663 mg/g. He underwent a colonoscopy which showed diffuse moderate inflammation and friability throughout the entire colon and terminal ileum. Biopsies demonstrated active chronic colitis and ileitis (cryptitis, crypt abscess formation, PMNs in lamina propria, minimal glandular architectural distortion), described as inflammatory bowel disease-like, most akin to ulcerative colitis. After discussions with oncology, the decision was made to hold his Obinutuzumab for likely monoclonal antibody induced colitis. His left lower quadrant pain improved, and he had a significant decrease in his fecal calprotectin to 397 mg/g.
Discussion: It is established that Obinutuzumab has several side effects including diarrhea. Generally, these patients are treated supportively and do not undergo colonoscopy. Here we presented a case of CD20 inhibitor induced colitis, confirmed with histology. This disease may have been missed if the patient was not due for colorectal cancer screening. This could indicate that CD20 induced colitis is underdiagnosed. More research is needed to establish colitis as a potential adverse effect of Obinutuzumab, and to clarify the role of endoscopic evaluation in these patients.
Disclosures:
Sean Sullivan, DO1, Julie Sullivan, DO2, Steven Stanek, MD3. P3778 - Obinutuzumab: A Rare Cause of Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Bryn Mawr, Norristown, PA; 2Einstein Medical Center, Norristown, PA; 3Einstein Healthcare Network, East Norriton, PA
Introduction: Colitis is an increasingly recognized adverse effect of many chemotherapeutic agents. Cytotoxic agents and checkpoint inhibitor immunotherapies have been well documented to cause colitis, leading to an increasing number of hospital admissions and increased morbidity. Here we present a case of colitis secondary to Obinutuzumab, a CD 20 monoclonal antibody used to treat B-cell mediated malignancies, a side effect rarely reported in the literature.
Case Description/Methods: Our patient is a 62-year-old male with past medical history of gastroesophageal reflux disease, hypertension, and follicular lymphoma of the head and neck treated with resection. He developed recurrent follicular lymphoma 15 years after initial diagnosis. He was started on combination therapy with bendamustine and Obinutuzumab and then transitioned to maintenance therapy with Obinutuzumab every two months. After 18 months of treatment, he was referred to gastroenterology for complaints of persistent left lower quadrant abdominal pain and routine colorectal cancer screening, at that time, he was noted to have elevated fecal calprotectin 663 mg/g. He underwent a colonoscopy which showed diffuse moderate inflammation and friability throughout the entire colon and terminal ileum. Biopsies demonstrated active chronic colitis and ileitis (cryptitis, crypt abscess formation, PMNs in lamina propria, minimal glandular architectural distortion), described as inflammatory bowel disease-like, most akin to ulcerative colitis. After discussions with oncology, the decision was made to hold his Obinutuzumab for likely monoclonal antibody induced colitis. His left lower quadrant pain improved, and he had a significant decrease in his fecal calprotectin to 397 mg/g.
Discussion: It is established that Obinutuzumab has several side effects including diarrhea. Generally, these patients are treated supportively and do not undergo colonoscopy. Here we presented a case of CD20 inhibitor induced colitis, confirmed with histology. This disease may have been missed if the patient was not due for colorectal cancer screening. This could indicate that CD20 induced colitis is underdiagnosed. More research is needed to establish colitis as a potential adverse effect of Obinutuzumab, and to clarify the role of endoscopic evaluation in these patients.
Disclosures:
Sean Sullivan indicated no relevant financial relationships.
Julie Sullivan indicated no relevant financial relationships.
Steven Stanek indicated no relevant financial relationships.
Sean Sullivan, DO1, Julie Sullivan, DO2, Steven Stanek, MD3. P3778 - Obinutuzumab: A Rare Cause of Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
