Tuesday Poster Session
Category: Colon
P3663 - Pembrolizumab Predominantly Causes Microscopic Colitis That Responds to Oral Budesonide in Patients Who Develop Immune Checkpoint Inhibitor Colitis: A Retrospective Single Site Observational Study
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Pearl Aggarwal, MD
University Hospitals St. John Medical Center
Westlake, OH
Presenting Author(s)
Pearl Aggarwal, MD1, Jaime A. Perez, PhD2, Jennifer E Murphy, PhD2, Saleem Chowdhry, MD1
1University Hospitals St. John Medical Center, Westlake, OH; 2University Hospitals Clinical Research Center, Cleveland, OH
Introduction: Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, but adverse events like colitis pose management challenges. This study characterizes ICI-related colitis subtypes, focusing on Pembrolizumab's impact on the occurrence of Microscopic colitis (MC)-like ICI colitis, vital for improved patient outcomes.
Methods: A retrospective chart review analyzed patients with post-ICI diarrhea (Pembrolizumab, Nivolumab, and Ipilimumab) from 12/2017 to 12/2022. Patients were categorized into MC-like and non-MC-like ICI colitis. Subgroups included Pembrolizumab-mediated MC-like ICI colitis (A), non-Pembrolizumab mediated MC-like ICI colitis (B), Pembrolizumab mediated non-MC-like ICI colitis (C), and non-Pembrolizumab mediated non-MC-like ICI colitis (D). Non Pembrolizumab ICI include -Nivolumab and Ipilimumab. Data was analyzed using chi-square, Kruskal-Wallis chi-squared, and Fisher Exact tests.
Results: Out of 113 patients who had a diagnosis of diarrhea and recent ICI use, 30 patients (Pembrolizumab: 12, Non-Pembrolizumab: 18) were diagnosed specifically (based on current AGA and ASCO guidelines) with ICI colitis based on symptoms, lab results, and colonoscopy/flexible sigmoidoscopy. 18 (Pembrolizumab: 11 and Non Pembrolizumab: 7)/30 patients underwent a colonoscopy/flexible sigmoidoscopy.
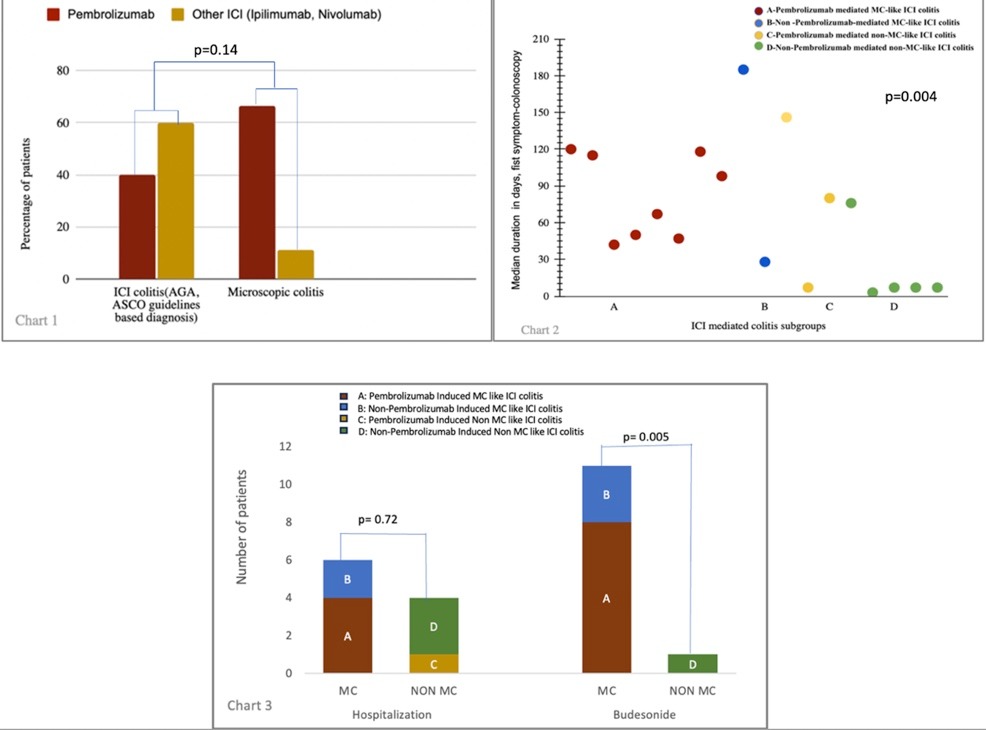
Pembrolizumab caused MC-like ICI colitis (Group A) in 66.67% (8/12) vs non-Pembrolizumab (Group B) 11% (2/18), p= 0.004, Chart 1.
The time interval between symptom onset and colonoscopy was longer in Groups A, B, and C (82, 106, and 80 days) vs. Group D (7 days), p= 0.14, Chart 2.
The hospitalization rate was higher in MC-like ICI colitis (Groups A plus B) (60% v/s 50%), p= 0.72. All patients with MC-like ICI colitis responded completely to oral Budesonide, p= 0.005, Chart 3.
MC-like ICI colitis was common in males (66.6%).
Discussion: AGA and the American Society of Clinical Oncology (ASCO) recommend colonoscopy only in patients with moderate to severe symptoms and do not differentiate between ICIs. Only 2 published retrospective studies have suggested an MC-like profile on histopathology in patients with ICI colitis. Our study is the 1st to show a significantly higher risk of this subtype with Pembrolizumab. In addition, our data analysis points towards a higher hospitalization rate in patients with MC-like ICI colitis and a delay in endoscopic intervention for diagnosis, thus emphasizing the need for prospective research and refined guidelines to improve histopathological-based management.
Disclosures:
Pearl Aggarwal, MD1, Jaime A. Perez, PhD2, Jennifer E Murphy, PhD2, Saleem Chowdhry, MD1. P3663 - Pembrolizumab Predominantly Causes Microscopic Colitis That Responds to Oral Budesonide in Patients Who Develop Immune Checkpoint Inhibitor Colitis: A Retrospective Single Site Observational Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University Hospitals St. John Medical Center, Westlake, OH; 2University Hospitals Clinical Research Center, Cleveland, OH
Introduction: Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, but adverse events like colitis pose management challenges. This study characterizes ICI-related colitis subtypes, focusing on Pembrolizumab's impact on the occurrence of Microscopic colitis (MC)-like ICI colitis, vital for improved patient outcomes.
Methods: A retrospective chart review analyzed patients with post-ICI diarrhea (Pembrolizumab, Nivolumab, and Ipilimumab) from 12/2017 to 12/2022. Patients were categorized into MC-like and non-MC-like ICI colitis. Subgroups included Pembrolizumab-mediated MC-like ICI colitis (A), non-Pembrolizumab mediated MC-like ICI colitis (B), Pembrolizumab mediated non-MC-like ICI colitis (C), and non-Pembrolizumab mediated non-MC-like ICI colitis (D). Non Pembrolizumab ICI include -Nivolumab and Ipilimumab. Data was analyzed using chi-square, Kruskal-Wallis chi-squared, and Fisher Exact tests.
Results: Out of 113 patients who had a diagnosis of diarrhea and recent ICI use, 30 patients (Pembrolizumab: 12, Non-Pembrolizumab: 18) were diagnosed specifically (based on current AGA and ASCO guidelines) with ICI colitis based on symptoms, lab results, and colonoscopy/flexible sigmoidoscopy. 18 (Pembrolizumab: 11 and Non Pembrolizumab: 7)/30 patients underwent a colonoscopy/flexible sigmoidoscopy.
Pembrolizumab caused MC-like ICI colitis (Group A) in 66.67% (8/12) vs non-Pembrolizumab (Group B) 11% (2/18), p= 0.004, Chart 1.
The time interval between symptom onset and colonoscopy was longer in Groups A, B, and C (82, 106, and 80 days) vs. Group D (7 days), p= 0.14, Chart 2.
The hospitalization rate was higher in MC-like ICI colitis (Groups A plus B) (60% v/s 50%), p= 0.72. All patients with MC-like ICI colitis responded completely to oral Budesonide, p= 0.005, Chart 3.
MC-like ICI colitis was common in males (66.6%).
Discussion: AGA and the American Society of Clinical Oncology (ASCO) recommend colonoscopy only in patients with moderate to severe symptoms and do not differentiate between ICIs. Only 2 published retrospective studies have suggested an MC-like profile on histopathology in patients with ICI colitis. Our study is the 1st to show a significantly higher risk of this subtype with Pembrolizumab. In addition, our data analysis points towards a higher hospitalization rate in patients with MC-like ICI colitis and a delay in endoscopic intervention for diagnosis, thus emphasizing the need for prospective research and refined guidelines to improve histopathological-based management.

Figure: Charts
Disclosures:
Pearl Aggarwal indicated no relevant financial relationships.
Jaime Perez indicated no relevant financial relationships.
Jennifer E Murphy indicated no relevant financial relationships.
Saleem Chowdhry indicated no relevant financial relationships.
Pearl Aggarwal, MD1, Jaime A. Perez, PhD2, Jennifer E Murphy, PhD2, Saleem Chowdhry, MD1. P3663 - Pembrolizumab Predominantly Causes Microscopic Colitis That Responds to Oral Budesonide in Patients Who Develop Immune Checkpoint Inhibitor Colitis: A Retrospective Single Site Observational Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
