Monday Poster Session
Category: Liver
P3091 - A Novel Case of Pyridostigmine Induced Liver Injury
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Laura DiBenedetto, DO
New York University Langone Health
East Setauket, NY
Presenting Author(s)
Laura DiBenedetto, DO1, Aniza Nasir, BS, MS2, Vincent Wong, MD3, Bilal Asif, MD4
1New York University Langone Health, East Setauket, NY; 2Rutgers Biomedical and Health Sciences, Newark, NJ; 3NYU Langone Health, Mineola, NY; 4New York University Langone Health, Mineola, NY
Introduction: Drug-induced liver Injury (DILI) describes acute hepatotoxicity from offending drugs or their metabolites. Most cases resolve quickly after the medication is discontinued. The diagnosis of DILI is typically made by establishing a relationship between drug exposure and the development of signs and symptoms of liver injury. It remains a diagnosis of exclusion, requiring ruling out of infectious, autoimmune, and other causes.
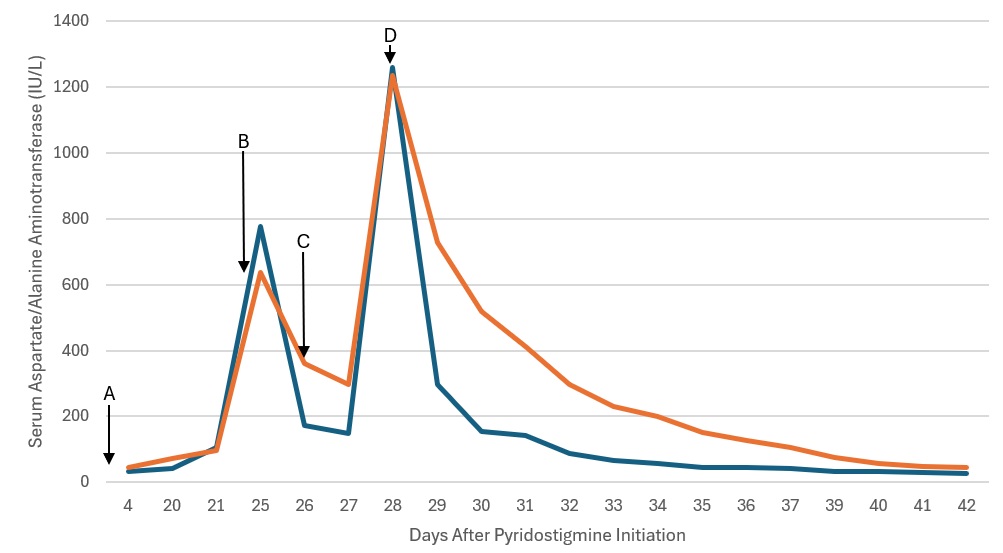
Case Description/Methods: A 72-year-old male with history significant for left femoral deep vein thrombosis and pulmonary embolism on Eliquis, chronic kidney disease, dysphagia, and hypertension presented for evaluation of achalasia. He underwent peroral endoscopic myotomy and post-operatively developed Ogilvie’s Syndrome. His intestinal dysmotility didn’t respond to multiple prokinetic agents, leading to initiation of pyridostigmine therapy with dose adjustments. Liver enzymes were noted to be elevated after starting the medication. Imaging results including magnetic resonance cholangiopancreatography and computerized axial tomography of the abdomen were unrevealing. Serological, infectious, and autoimmune workup was largely negative, leading to pyridostigmine discontinuation. (Table 1.) After an improvement in transaminases, from about 700IU/L to around 400IU/L, Pyridostigmine was restarted. Within 48 hours, significant transaminase elevation, INR elevation, and worsening mental status were noted. Pyridostigmine was then discontinued, with major improvement in 24 hours, and normalization of transaminases within days. (Figure 1.)
Discussion: Pyridostigmine is an acetylcholinesterase inhibitor that increases extracellular acetylcholine levels in the neuromuscular junction by impairing its breakdown. It is frequently used as a potential treatment modality in many etiologies of constipation, such as colonic pseudo-obstruction. While it is thought to exert its effects by increasing gastrointestinal motility, there remains a paucity of literature on Pyridostigmine-induced liver injury. In the present case, a patient with no other hepatic medical history and negative serological workup made a full recovery immediately after discontinuation of Pyridostigmine. Clinicians should be aware of the risk of DILI when using Pyridostigmine and evaluate patients’ risk appropriately.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Laura DiBenedetto, DO1, Aniza Nasir, BS, MS2, Vincent Wong, MD3, Bilal Asif, MD4. P3091 - A Novel Case of Pyridostigmine Induced Liver Injury, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1New York University Langone Health, East Setauket, NY; 2Rutgers Biomedical and Health Sciences, Newark, NJ; 3NYU Langone Health, Mineola, NY; 4New York University Langone Health, Mineola, NY
Introduction: Drug-induced liver Injury (DILI) describes acute hepatotoxicity from offending drugs or their metabolites. Most cases resolve quickly after the medication is discontinued. The diagnosis of DILI is typically made by establishing a relationship between drug exposure and the development of signs and symptoms of liver injury. It remains a diagnosis of exclusion, requiring ruling out of infectious, autoimmune, and other causes.
Case Description/Methods: A 72-year-old male with history significant for left femoral deep vein thrombosis and pulmonary embolism on Eliquis, chronic kidney disease, dysphagia, and hypertension presented for evaluation of achalasia. He underwent peroral endoscopic myotomy and post-operatively developed Ogilvie’s Syndrome. His intestinal dysmotility didn’t respond to multiple prokinetic agents, leading to initiation of pyridostigmine therapy with dose adjustments. Liver enzymes were noted to be elevated after starting the medication. Imaging results including magnetic resonance cholangiopancreatography and computerized axial tomography of the abdomen were unrevealing. Serological, infectious, and autoimmune workup was largely negative, leading to pyridostigmine discontinuation. (Table 1.) After an improvement in transaminases, from about 700IU/L to around 400IU/L, Pyridostigmine was restarted. Within 48 hours, significant transaminase elevation, INR elevation, and worsening mental status were noted. Pyridostigmine was then discontinued, with major improvement in 24 hours, and normalization of transaminases within days. (Figure 1.)
Discussion: Pyridostigmine is an acetylcholinesterase inhibitor that increases extracellular acetylcholine levels in the neuromuscular junction by impairing its breakdown. It is frequently used as a potential treatment modality in many etiologies of constipation, such as colonic pseudo-obstruction. While it is thought to exert its effects by increasing gastrointestinal motility, there remains a paucity of literature on Pyridostigmine-induced liver injury. In the present case, a patient with no other hepatic medical history and negative serological workup made a full recovery immediately after discontinuation of Pyridostigmine. Clinicians should be aware of the risk of DILI when using Pyridostigmine and evaluate patients’ risk appropriately.

Figure: Figure 1. Liver Injury with Pyridostigmine Challenge and Rechallenge. AST – Blue, ALT – Orange. A. Day 1 - Starting Dose 60 mg three times daily, B. Day 24 - Pyridostigmine Held, C. Day 26 - Pyridostigmine Restarted 30 mg three times daily. D. Day 28 - Pyridostigmine stopped.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Laura DiBenedetto indicated no relevant financial relationships.
Aniza Nasir indicated no relevant financial relationships.
Vincent Wong indicated no relevant financial relationships.
Bilal Asif indicated no relevant financial relationships.
Laura DiBenedetto, DO1, Aniza Nasir, BS, MS2, Vincent Wong, MD3, Bilal Asif, MD4. P3091 - A Novel Case of Pyridostigmine Induced Liver Injury, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
