Monday Poster Session
Category: IBD
P2726 - Second Trial of Induction of Mesalamine in Ulcerative Colitis Causing Acute Interstitial Nephritis: A Case Report
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
- DB
Dhanushya Battepati, MD
Baylor Scott & White Medical Center
Temple, TX
Presenting Author(s)
Dhanushya Battepati, MD, Lancaster Weld, DO, Raymond Duggan, DO
Baylor Scott & White Medical Center, Temple, TX
Introduction: Mesalamine is commonly used as a first line treatment for Ulcerative Colitis (UC). Although it is a relatively safe drug to use to treat UC, side effects of mesalamine described in the literature include symptoms mimicking an acute flare of UC, drug allergy, DRESS syndrome or mesalamine induced lupus. Renal impairment, although rare, is a side effect that may be detrimental if unrecognized and not promptly treated.
Case Description/Methods: We report a case of a 24-year-old female with a diagnosis of UC treated with Lialda (mesalamine) who was admitted to our hospital for acute kidney injury. Two years prior to presenting to the hospital, the patient had been on Lialda, but self-discontinued it due to symptom resolution. Due to recurrence of symptoms two years later, she restarted the medication. Shortly after restarting Lialda, it was noted that the patient had elevated creatinine levels. Her baseline creatinine levels were 0.94-1.2. Her initial elevated creatinine level was 1.59 and increased to 2.44 four months later.
The Nephrology service was consulted and had a differential diagnosis which included: medication-induced AIN, sarcoidosis, autoimmune etiologies and tuberculosis. A renal biopsy was performed for a definitive diagnosis which showed non-caseating granulomas, a characteristic of AIN. The underlying cause for AIN was attributed to Lialda, which was immediately discontinued. The patient was prescribed a prednisone taper over two months to treat the AIN. Despite the side effects she experienced from the steroid taper, her symptoms of UC improved. After a repeat outpatient colonoscopy, she then was initiated on Entyvio (vedolizumab). Her creatinine levels returned to her baseline.
Discussion: This case presentation is unique in that the patient did not have any signs of kidney impairment when she was first started on Lialda. Most cases of AIN are seen within the first 6 months to 1 year of initiation of mesalamine. Among the 5-Aminosalicylic acid (5-ASA) medications that induced AIN, a case series of 42 patients with IBD demonstrated mesalamine to be the most common. It is imperative that kidney function is monitored frequently for patients on mesalamine. The medication should be abruptly discontinued if there is a concern for AIN. The concern for mesalamine-induced AIN should be very high, especially in young patients who do not have a medical history that could contribute to a rise in creatinine, such as hypertension or diabetes mellitus.
Disclosures:
Dhanushya Battepati, MD, Lancaster Weld, DO, Raymond Duggan, DO. P2726 - Second Trial of Induction of Mesalamine in Ulcerative Colitis Causing Acute Interstitial Nephritis: A Case Report, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Baylor Scott & White Medical Center, Temple, TX
Introduction: Mesalamine is commonly used as a first line treatment for Ulcerative Colitis (UC). Although it is a relatively safe drug to use to treat UC, side effects of mesalamine described in the literature include symptoms mimicking an acute flare of UC, drug allergy, DRESS syndrome or mesalamine induced lupus. Renal impairment, although rare, is a side effect that may be detrimental if unrecognized and not promptly treated.
Case Description/Methods: We report a case of a 24-year-old female with a diagnosis of UC treated with Lialda (mesalamine) who was admitted to our hospital for acute kidney injury. Two years prior to presenting to the hospital, the patient had been on Lialda, but self-discontinued it due to symptom resolution. Due to recurrence of symptoms two years later, she restarted the medication. Shortly after restarting Lialda, it was noted that the patient had elevated creatinine levels. Her baseline creatinine levels were 0.94-1.2. Her initial elevated creatinine level was 1.59 and increased to 2.44 four months later.
The Nephrology service was consulted and had a differential diagnosis which included: medication-induced AIN, sarcoidosis, autoimmune etiologies and tuberculosis. A renal biopsy was performed for a definitive diagnosis which showed non-caseating granulomas, a characteristic of AIN. The underlying cause for AIN was attributed to Lialda, which was immediately discontinued. The patient was prescribed a prednisone taper over two months to treat the AIN. Despite the side effects she experienced from the steroid taper, her symptoms of UC improved. After a repeat outpatient colonoscopy, she then was initiated on Entyvio (vedolizumab). Her creatinine levels returned to her baseline.
Discussion: This case presentation is unique in that the patient did not have any signs of kidney impairment when she was first started on Lialda. Most cases of AIN are seen within the first 6 months to 1 year of initiation of mesalamine. Among the 5-Aminosalicylic acid (5-ASA) medications that induced AIN, a case series of 42 patients with IBD demonstrated mesalamine to be the most common. It is imperative that kidney function is monitored frequently for patients on mesalamine. The medication should be abruptly discontinued if there is a concern for AIN. The concern for mesalamine-induced AIN should be very high, especially in young patients who do not have a medical history that could contribute to a rise in creatinine, such as hypertension or diabetes mellitus.

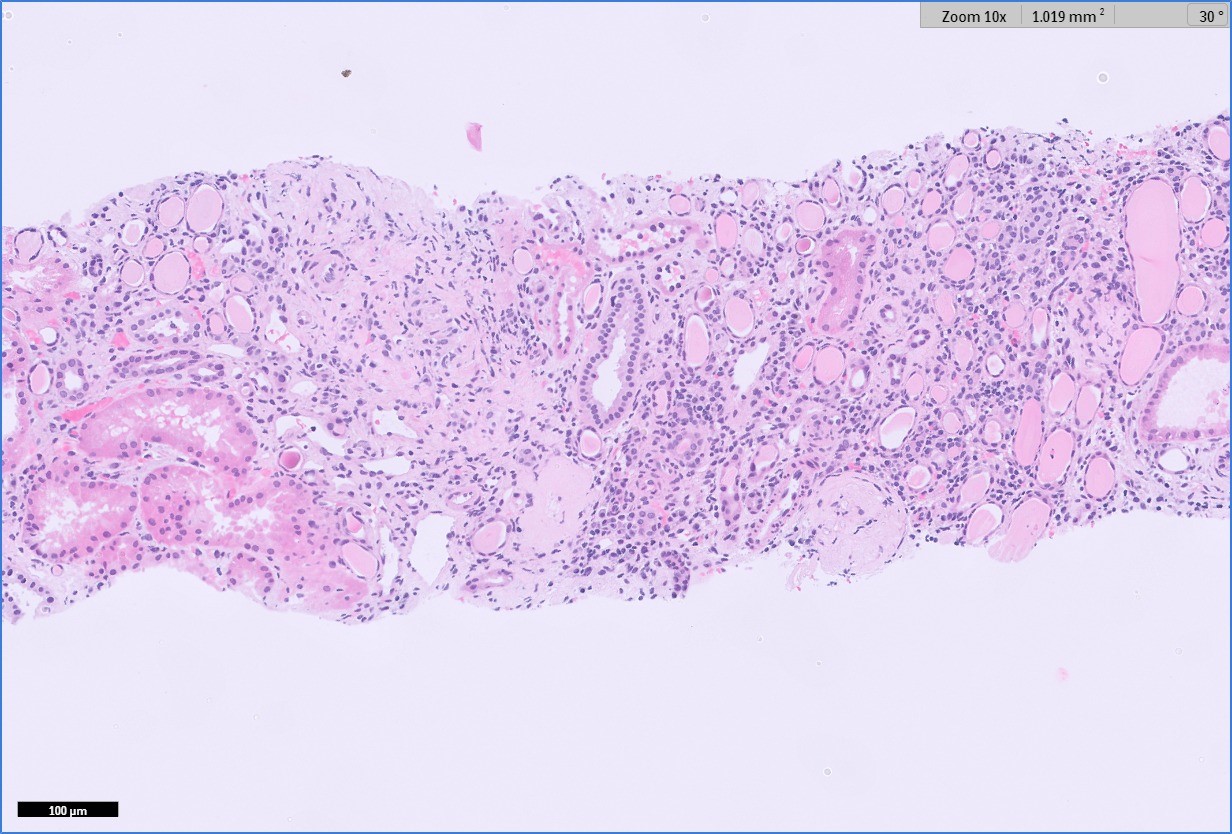
Figure: H&E stains show non-necrotizing granulomatous inflammation and tubulointerstitial injury. There is moderate mixed interstitial inflammatory infiltrate composed of lymphocytes, aggregates of plasma cells and few aggregates of eosinophils (100X).
Disclosures:
Dhanushya Battepati indicated no relevant financial relationships.
Lancaster Weld indicated no relevant financial relationships.
Raymond Duggan indicated no relevant financial relationships.
Dhanushya Battepati, MD, Lancaster Weld, DO, Raymond Duggan, DO. P2726 - Second Trial of Induction of Mesalamine in Ulcerative Colitis Causing Acute Interstitial Nephritis: A Case Report, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
