Monday Poster Session
Category: IBD
P2651 - Need to Improve Awareness for Cervical Dysplasia Screening in Immunosuppressed Patients With Inflammatory Bowel Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

- DB
Dhanushya Battepati, MD
Baylor Scott & White Medical Center
Temple, TX
Presenting Author(s)
Phi Tran, DO, Dhanushya Battepati, MD, Collin Telchik, MD, Britney Constans, DO, Michelle Harris, MD, Alexis Bejcek, MD, Christopher Johnson, MD
Baylor Scott & White Medical Center, Temple, TX
Introduction: Despite the increased risk for cervical cancer in patients with inflammatory bowel disease (IBD) on immunosuppressants, screening guidelines appear to vary amongst various medical organizations. The American College of Obstetrics and Gynecology (ACOG) supports the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines for cervical cancer screening in immunosuppressed women without HIV (1). They recommend that women with IBD on immunosuppressive drugs should be screened under high-risk guidelines, or yearly, as opposed to average-risk screening recommendation of a 3 to 5-year interval. Our study looks at the proportion of patients from 2016-2022 with IBD on immunosuppressive therapy screened for cervical dysplasia.
Methods: This retrospective cohort study was conducted at Baylor Scott and White Medical Center from 2016 to 2022. It focuses on women with IBD undergoing immunosuppressive therapy. The inclusion criteria were a diagnosis of UC or CD, female sex, age between 21 and 65, and a current history of immunosuppressive therapy. Immunosuppressive therapy was defined as at least three consecutive months of steroid use or one year of immunomodulator or biologic therapy. For cervical dysplasia screening, average-risk guidelines recommended pap smears every 3-5 years, while high-risk guidelines suggested annual screening.
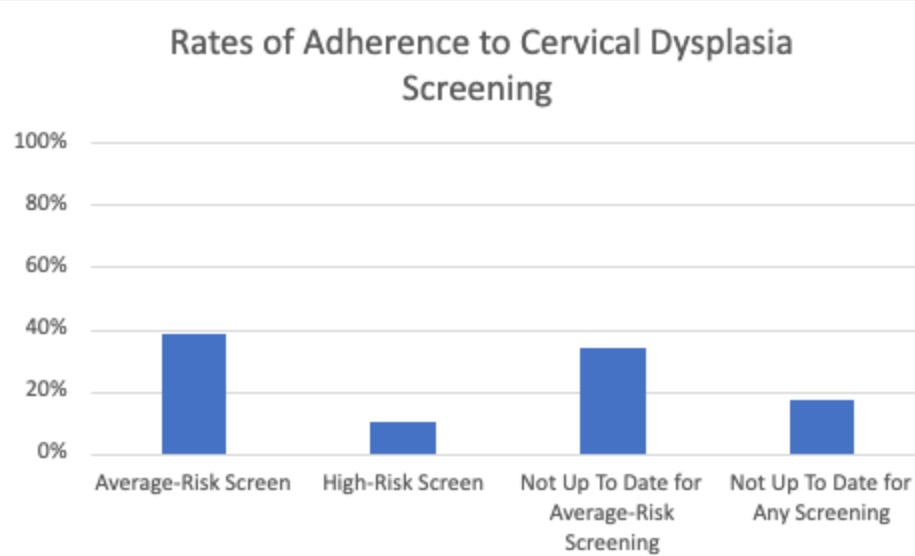
Results: Out of the 191 patients who met the inclusion criteria, 82.7% (n = 158) were screened for human papillomavirus (HPV). Among these patients, 38.7% (n = 74) were screened based on general population guidelines, while only 9.9% (n = 19) were screened following high-risk guidelines. However, 34% (n = 65) were not up to date according to the average risk guidelines, and 17.3% (n = 33) had never received any screening at all. Among the patients with pap smear pathology available in our EMR, 8.6% (n = 8) tested positive for HPV, and 11.9% (n = 11) had abnormal cytology.
Discussion: The screening rates for cervical dysplasia were low for both average-risk and high-risk individuals according to the guidelines recommended by ACOG. Although there is ample evidence to suggest a higher risk of cervical cancer in patients with IBD on immunosuppressants compared to the general population, there is no consensus among primary care physicians, obstetricians/gynecologists, and gastroenterologists. Increased efforts should be made to increase awareness of HPV screening, especially in the high-risk populations.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Phi Tran, DO, Dhanushya Battepati, MD, Collin Telchik, MD, Britney Constans, DO, Michelle Harris, MD, Alexis Bejcek, MD, Christopher Johnson, MD. P2651 - Need to Improve Awareness for Cervical Dysplasia Screening in Immunosuppressed Patients With Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Baylor Scott & White Medical Center, Temple, TX
Introduction: Despite the increased risk for cervical cancer in patients with inflammatory bowel disease (IBD) on immunosuppressants, screening guidelines appear to vary amongst various medical organizations. The American College of Obstetrics and Gynecology (ACOG) supports the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines for cervical cancer screening in immunosuppressed women without HIV (1). They recommend that women with IBD on immunosuppressive drugs should be screened under high-risk guidelines, or yearly, as opposed to average-risk screening recommendation of a 3 to 5-year interval. Our study looks at the proportion of patients from 2016-2022 with IBD on immunosuppressive therapy screened for cervical dysplasia.
Methods: This retrospective cohort study was conducted at Baylor Scott and White Medical Center from 2016 to 2022. It focuses on women with IBD undergoing immunosuppressive therapy. The inclusion criteria were a diagnosis of UC or CD, female sex, age between 21 and 65, and a current history of immunosuppressive therapy. Immunosuppressive therapy was defined as at least three consecutive months of steroid use or one year of immunomodulator or biologic therapy. For cervical dysplasia screening, average-risk guidelines recommended pap smears every 3-5 years, while high-risk guidelines suggested annual screening.
Results: Out of the 191 patients who met the inclusion criteria, 82.7% (n = 158) were screened for human papillomavirus (HPV). Among these patients, 38.7% (n = 74) were screened based on general population guidelines, while only 9.9% (n = 19) were screened following high-risk guidelines. However, 34% (n = 65) were not up to date according to the average risk guidelines, and 17.3% (n = 33) had never received any screening at all. Among the patients with pap smear pathology available in our EMR, 8.6% (n = 8) tested positive for HPV, and 11.9% (n = 11) had abnormal cytology.
Discussion: The screening rates for cervical dysplasia were low for both average-risk and high-risk individuals according to the guidelines recommended by ACOG. Although there is ample evidence to suggest a higher risk of cervical cancer in patients with IBD on immunosuppressants compared to the general population, there is no consensus among primary care physicians, obstetricians/gynecologists, and gastroenterologists. Increased efforts should be made to increase awareness of HPV screening, especially in the high-risk populations.

Figure: Figure 1: Adherence to HPV Screening amongst Females with IBD on Chronic Immunosuppression
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Phi Tran indicated no relevant financial relationships.
Dhanushya Battepati indicated no relevant financial relationships.
Collin Telchik indicated no relevant financial relationships.
Britney Constans indicated no relevant financial relationships.
Michelle Harris indicated no relevant financial relationships.
Alexis Bejcek indicated no relevant financial relationships.
Christopher Johnson indicated no relevant financial relationships.
Phi Tran, DO, Dhanushya Battepati, MD, Collin Telchik, MD, Britney Constans, DO, Michelle Harris, MD, Alexis Bejcek, MD, Christopher Johnson, MD. P2651 - Need to Improve Awareness for Cervical Dysplasia Screening in Immunosuppressed Patients With Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
