Monday Poster Session
Category: IBD
P2580 - The Efficacy of Maintenance Treatment with Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis: Phase 3 QUASAR Maintenance Study Results at Week 44 by Biologic/Janus Kinase History
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Jessica Allegretti, MD, MPH, FACG
Brigham and Women’s Hospital, Harvard Medical School
Boston, MA
Presenting Author(s)
Jessica R. Allegretti, MD, MPH, FACG1, Julián Panés, MD2, Laurent Peyrin-Biroulet, MD, PhD3, Bruce E.. Sands, MD, FACG4, Shadi Yarandi, PhD5, Kuan-Hsiang G. Huang, MD, PhD6, Matthew Germinaro, MD6, Jia Zhan, PhD6, Hongyan Zhang, PhD6, Jakob Begun, MD, PhD7, Jarosław Kierkuś, MD, PhD8, Tetiana Kravchenko, MD, PhD9, Tadakazu Hisamatsu, MD, PhD10, David T. Rubin, MD, FACG11, Brian Bressler, MS, MD12, Axel Dignass, MD, PhD13
1Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 3INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 4Icahn School of Medicine at Mount Sinai, New York, NY; 5Janssen Research & Development, LLC, Spring House, PA; 6Janssen Research and Development, Spring House, PA; 7Mater Hospital, South Brisbane, Queensland, Australia; 8The Children's Memorial Health Institute, Warsaw, Mazowieckie, Poland; 9National Medical University, Kyiv, Kyyivs'ka Oblast', Ukraine; 10Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 11University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 12University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada; 13Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany
Introduction: The Phase 3 QUASAR Maintenance Study evaluated the efficacy of maintenance treatment with subcutaneous (SC) guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor, in patients (pts) with ulcerative colitis (UC) who achieved clinical response following IV GUS induction treatment. Here, we report Week (Wk) 44 efficacy and safety results for GUS treatment compared with withdrawal (placebo) by history of treatment with biologics/Janus kinase inhibitors (BIO/JAK).
Methods: At maintenance baseline, clinical responders to 12 wks of GUS IV induction from QUASAR Phase 2b and Phase 3 induction studies (NCT04033445) were randomized 1:1:1 to GUS 200mg SC q4w, GUS 100mg SC q8w, or PBO (GUS withdrawal). The primary analysis population included randomized and treated pts in the Maintenance Study with a modified Mayo score of 5-9 at induction baseline. Key clinical, histologic, and endoscopic efficacy endpoints and safety events were evaluated through Wk44.
Results: Of the 568 pts included, 240 (42.3%) had a history of inadequate response or intolerance to BIO/JAK (BIO/JAK-IR) and 309 (54.4%) were BIO/JAK naïve, At induction baseline, BIO/JAK-IR pts had longer disease duration (mean 9.94 vs 6.25 yrs) and more severe endoscopic disease (Mayo endoscopic subscore[MES]=3, 79.6% vs 56.7%) compared with pts with no BIO/JAK history.
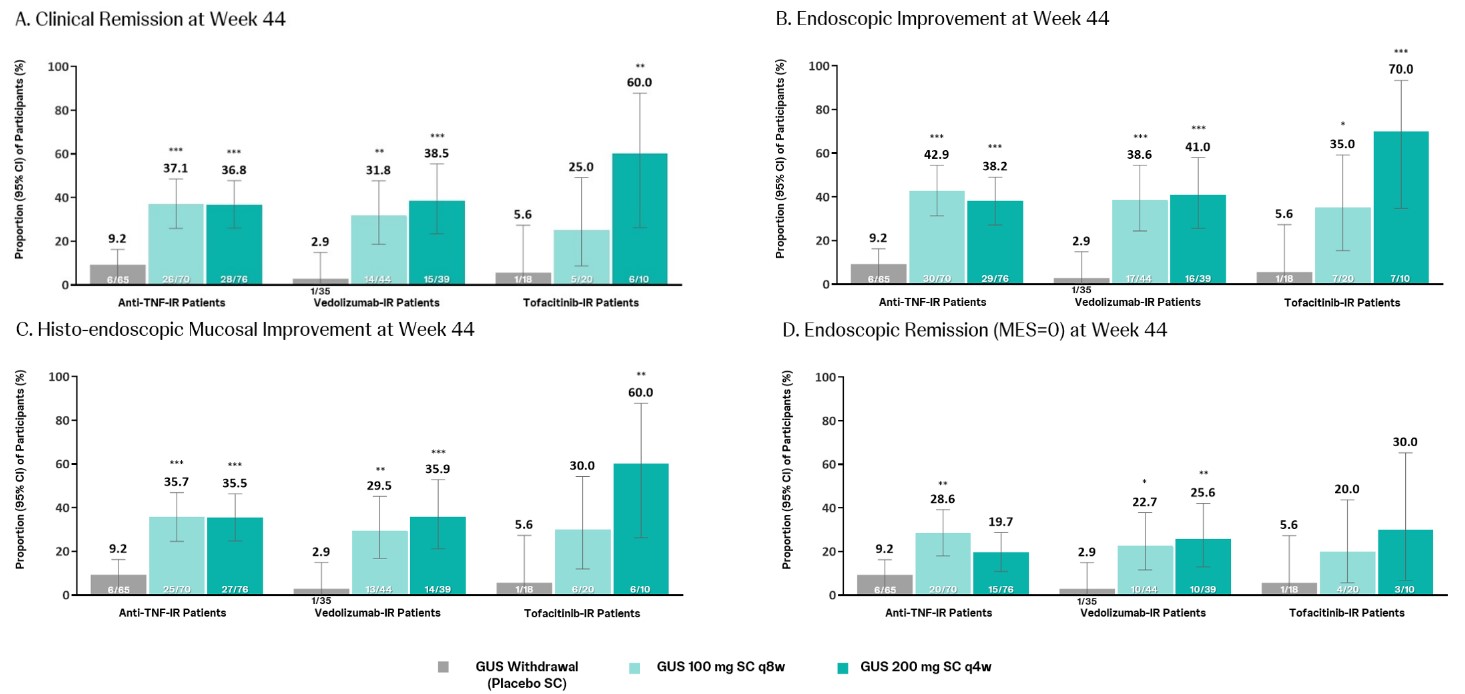
Among both the BIO/JAK-IR and naïve subpopulations, higher proportions of GUS-treated pts achieved the key efficacy endpoints at Wk44 compared with withdrawal pts (Table 1). The proportions of pts who met the key endpoints at Wk44 were generally higher in the BIO/JAK naïve subpopulation compared with the BIO/JAK-IR subpopulation across treatment groups, however, treatment differences were generally greater in BIO/JAK-IR pts. Among the anti-TNF-IR, vedolizumab-IR, or tofacitinib-IR pts, higher proportions of GUS-treated pts achieved the key endpoints at Wk44 compared with withdrawal pts (Figure 1). Through Wk44, adverse events for the BIO/JAK-IR and BIO/JAK naïve subpopulations were consistent with the overall population, and no new safety concerns were identified.
Discussion: Maintenance treatment with both GUS dosing regimens resulted in greater improvements compared with placebo withdrawal across key clinical, endoscopic, and histologic endpoints at Wk44 regardless of prior history with biologics/JAK inhibitors. Safety results for both GUS maintenance dose regimens were comparable across subpopulations by treatment history and consistent with the known safety profile of GUS.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jessica R. Allegretti, MD, MPH, FACG1, Julián Panés, MD2, Laurent Peyrin-Biroulet, MD, PhD3, Bruce E.. Sands, MD, FACG4, Shadi Yarandi, PhD5, Kuan-Hsiang G. Huang, MD, PhD6, Matthew Germinaro, MD6, Jia Zhan, PhD6, Hongyan Zhang, PhD6, Jakob Begun, MD, PhD7, Jarosław Kierkuś, MD, PhD8, Tetiana Kravchenko, MD, PhD9, Tadakazu Hisamatsu, MD, PhD10, David T. Rubin, MD, FACG11, Brian Bressler, MS, MD12, Axel Dignass, MD, PhD13. P2580 - The Efficacy of Maintenance Treatment with Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis: Phase 3 QUASAR Maintenance Study Results at Week 44 by Biologic/Janus Kinase History, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 3INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 4Icahn School of Medicine at Mount Sinai, New York, NY; 5Janssen Research & Development, LLC, Spring House, PA; 6Janssen Research and Development, Spring House, PA; 7Mater Hospital, South Brisbane, Queensland, Australia; 8The Children's Memorial Health Institute, Warsaw, Mazowieckie, Poland; 9National Medical University, Kyiv, Kyyivs'ka Oblast', Ukraine; 10Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 11University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 12University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada; 13Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany
Introduction: The Phase 3 QUASAR Maintenance Study evaluated the efficacy of maintenance treatment with subcutaneous (SC) guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor, in patients (pts) with ulcerative colitis (UC) who achieved clinical response following IV GUS induction treatment. Here, we report Week (Wk) 44 efficacy and safety results for GUS treatment compared with withdrawal (placebo) by history of treatment with biologics/Janus kinase inhibitors (BIO/JAK).
Methods: At maintenance baseline, clinical responders to 12 wks of GUS IV induction from QUASAR Phase 2b and Phase 3 induction studies (NCT04033445) were randomized 1:1:1 to GUS 200mg SC q4w, GUS 100mg SC q8w, or PBO (GUS withdrawal). The primary analysis population included randomized and treated pts in the Maintenance Study with a modified Mayo score of 5-9 at induction baseline. Key clinical, histologic, and endoscopic efficacy endpoints and safety events were evaluated through Wk44.
Results: Of the 568 pts included, 240 (42.3%) had a history of inadequate response or intolerance to BIO/JAK (BIO/JAK-IR) and 309 (54.4%) were BIO/JAK naïve, At induction baseline, BIO/JAK-IR pts had longer disease duration (mean 9.94 vs 6.25 yrs) and more severe endoscopic disease (Mayo endoscopic subscore[MES]=3, 79.6% vs 56.7%) compared with pts with no BIO/JAK history.
Among both the BIO/JAK-IR and naïve subpopulations, higher proportions of GUS-treated pts achieved the key efficacy endpoints at Wk44 compared with withdrawal pts (Table 1). The proportions of pts who met the key endpoints at Wk44 were generally higher in the BIO/JAK naïve subpopulation compared with the BIO/JAK-IR subpopulation across treatment groups, however, treatment differences were generally greater in BIO/JAK-IR pts. Among the anti-TNF-IR, vedolizumab-IR, or tofacitinib-IR pts, higher proportions of GUS-treated pts achieved the key endpoints at Wk44 compared with withdrawal pts (Figure 1). Through Wk44, adverse events for the BIO/JAK-IR and BIO/JAK naïve subpopulations were consistent with the overall population, and no new safety concerns were identified.
Discussion: Maintenance treatment with both GUS dosing regimens resulted in greater improvements compared with placebo withdrawal across key clinical, endoscopic, and histologic endpoints at Wk44 regardless of prior history with biologics/JAK inhibitors. Safety results for both GUS maintenance dose regimens were comparable across subpopulations by treatment history and consistent with the known safety profile of GUS.

Figure: Figure 1. Key endpoints at Maintenance Week 44 by history of anti-TNF, vedolizumab, or tofacitinib therapy: Primary analysis population.
*Nominal P<0.05. **Nominal P<0.01. ***Nominal P<0.001. Denominator is the number of pts who had inadequate response or intolerance to anti-TNFs, vedolizumab, or tofacitinib, regardless of other advanced therapies including adalimumab, golimumab, infliximab, tofacitinib, vedolizumab, and their biosimilars. Pts who had a prohibited change in UC medication, an ostomy or colectomy, a dose adjustment (including a sham dose adjustment) , or discontinued study agent due to lack of efficacy or an adverse event of worsening of UC or other reasons except for COVID-19 related reasons (excluding COVID-19 infection) or regional crisis in Russia and Ukraine prior to the Wk 44 visit were considered not to have achieved the endpoint. Pts who were missing one or more components pertaining to a specified endpoint at Wk 44 were considered not to have achieved the endpoint.
*Nominal P<0.05. **Nominal P<0.01. ***Nominal P<0.001. Denominator is the number of pts who had inadequate response or intolerance to anti-TNFs, vedolizumab, or tofacitinib, regardless of other advanced therapies including adalimumab, golimumab, infliximab, tofacitinib, vedolizumab, and their biosimilars. Pts who had a prohibited change in UC medication, an ostomy or colectomy, a dose adjustment (including a sham dose adjustment) , or discontinued study agent due to lack of efficacy or an adverse event of worsening of UC or other reasons except for COVID-19 related reasons (excluding COVID-19 infection) or regional crisis in Russia and Ukraine prior to the Wk 44 visit were considered not to have achieved the endpoint. Pts who were missing one or more components pertaining to a specified endpoint at Wk 44 were considered not to have achieved the endpoint.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jessica R. Allegretti: Abbvie – Consultant, Speakers Bureau. Artugen – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant. Finch Therapeutics – Consultant. Janssen – Consultant. Merck – Grant/Research Support. Pfizer – Consultant. Seres – Consultant.
Julián Panés: AbbVie – Consultant, Honorarium. Alimentiv – Advisory Committee/Board Member, Consultant, Honorarium. Athos – Consultant, Honorarium. Atomwise – Consultant, Honorarium. Boehringer Ingelheim – Consultant, Honorarium. Celsius – Consultant, Honorarium. Ferring – Consultant, Honorarium. Galapagos – Consultant, Honorarium. Genentech/Roche – Consultant, Honorarium. GlaxoSmithKline – Consultant, Honorarium. Janssen – Consultant, Honorarium. Mirum – Advisory Committee/Board Member, Consultant, honorarium. Nimbus – Consultant, Honorarium. Pfizer – Consultant, Honorarium. Progenity – Consultant, Honorarium. Prometheus – Consultant, Honorarium. Protagonist – Consultant, Honorarium. Revolo – Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. Sorriso – Advisory Committee/Board Member, Consultant, Honorarium. Surrozen – Advisory Committee/Board Member, Consultant, Honorarium. Takeda – Consultant, Honorarium. Wasserman – Consultant, Honorarium.
Laurent Peyrin-Biroulet: AbbVie – Grant/Research Support, Personal fees. Allergan – Personal Fees. Alma Bio Therapeutics – Personal Fees. Amgen – Personal Fees. Applied Molecular Transport – Personal Fees. Arena – Personal Fees. Biogen – Personal Fees. Boehringer Ingelheim – Personal Fees. Bristol Myers Squibb – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. CTMA – Stock Options. Enterome – Personal Fees. Enthera – Personal Fees. Ferring – Personal Fees. Fresenius Kabi – Personal Fees. Genentech – Personal Fees. Gilead – Personal Fees. Hikma – Personal Fees. InDex Pharmaceuticals – Personal Fees. Janssen – Personal Fees. Lilly – Personal Fees. MSD – Grant/Research Support, Personal Fees. Mylan – Personal Fees. Nestlé – Personal Fees. Norgine – Personal Fees. Oppilan Pharma – Personal Fees. OSE Immunotherapeutics – Personal Fees. Pfizer Inc – Personal Fees. Pharmacosmos – Fees. Samsung Bioepis – Personal Fees. Sandoz – Personal Fees. Sterna – Personal Fees. Sublimity Therapeutics – Personal Fees. Takeda – Grant/Research Support, Personal Fees. Tillotts – Personal Fees. Vifor – Personal Fees.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Shadi Yarandi: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Kuan-Hsiang G. Huang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Jia Zhan: Johnson & Johnson – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Jakob Begun: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Anatara – Consultant. Bristol Myers Squibb – Consultant. Chiesi – Consultant. DrFalk – Consultant. Ferring – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKlein – Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Microba – Consultant. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Jarosław Kierkuś: Bristol-Myers Squibb – Fees. Celltrion – Fees. Ferring – Fees. Fresenius – Fees. Janssen – Fees. Lilly – Fees. Nestlé – Fees.
Tetiana Kravchenko: Janssen – Consultant, Clinical Investigator.
Tadakazu Hisamatsu: AbbVie – Grant/Research Support, lecture fees. Bristol Myers Squibb – Consultant. Daiichi-Sankyo – Grant/Research Support. EA Pharma – Consultant, Grant/Research Support, lecture fees. Gilead Sciences – Consultant. Janssen – Consultant. JIMRO – Grant/Research Support. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, lecture fees. Mochida Pharmaceutical – Grant/Research Support. Nippon Kayaku – Grant/Research Support. Pfizer – Grant/Research Support. Takeda Pharmaceutical – Grant/Research Support, lecture fees.
David T. Rubin: AbbVie – Consultant. Altrubio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol-Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. Connect BioPharma – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Brian Bressler: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Alimentiv – Advisory Committee/Board Member. Allergan – Advisory Committee/Board Member. Amgen – Advisory Committee/Board Member, Grant/Research Support. AMT – Advisory Committee/Board Member. Bausch Health – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Grant/Research Support. Celgene – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Eupraxia – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. Genentech/Roche – Advisory Committee/Board Member, Grant/Research Support. Gilead – Advisory Committee/Board Member. GSK – Grant/Research Support. Iterative Scopes – Advisory Committee/Board Member. Jamp – Advisor or Review Panel Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Merck – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisor or Review Panel Member. Mylan – Advisor or Review Panel Member. Novartis – Advisory Committee/Board Member, Speakers Bureau. Organon – Advisory Committee/Board Member, Speakers Bureau. Pendopharm – Advisor or Review Panel Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Protagonist – Advisor or Review Panel Member. Qu Biologic – Grant/Research Support, Stock Options. Sandoz – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau. Viatris – Advisor or Review Panel Member.
Axel Dignass: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Speakers Bureau. High5MD – Speakers Bureau. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Lilly – Consultant. Materia Prima – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Pharmacosmos – Consultant. Roche – Consultant. Sandoz – Consultant, Speakers Bureau. Stada – Consultant. Takeda – Consultant, Payment for manuscript preparation, Speakers Bureau. Thieme – Payment for manuscript preparation. Tillotts – Consultant, Speakers Bureau. UniMed Verlag – Payment for manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Jessica R. Allegretti, MD, MPH, FACG1, Julián Panés, MD2, Laurent Peyrin-Biroulet, MD, PhD3, Bruce E.. Sands, MD, FACG4, Shadi Yarandi, PhD5, Kuan-Hsiang G. Huang, MD, PhD6, Matthew Germinaro, MD6, Jia Zhan, PhD6, Hongyan Zhang, PhD6, Jakob Begun, MD, PhD7, Jarosław Kierkuś, MD, PhD8, Tetiana Kravchenko, MD, PhD9, Tadakazu Hisamatsu, MD, PhD10, David T. Rubin, MD, FACG11, Brian Bressler, MS, MD12, Axel Dignass, MD, PhD13. P2580 - The Efficacy of Maintenance Treatment with Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis: Phase 3 QUASAR Maintenance Study Results at Week 44 by Biologic/Janus Kinase History, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
