Monday Poster Session
Category: General Endoscopy
P2387 - A Novel Protocol for Endoscope Handling and Disposal in the Setting of Creutzfeldt-Jakob and Other Prion Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Yara Salameh, MD
Mayo Foundation for Medical Education and Research
Rochester, MN
Presenting Author(s)
Yara Salameh, MD1, Lea Sayegh, MD1, Michelle Meyer, PhD2, Miranda Hamlin, MA2, Manik Aggarwal, MBBS2, Barham Abu Dayyeh, MD2, Nayantara Coelho-Prabhu, MD, FACG2, Vinay Chandrasekhara, MD2, Amanda M. Johnson, MD2, Ryan Law, DO2, John Martin, MD2, Bret Petersen, MD2, Andrew Storm, MD2
1Mayo Foundation for Medical Education and Research, Rochester, MN; 2Mayo Clinic, Rochester, MN
Introduction: Known as “slow virus” disease, Creutzfeldt-Jakob Disease (CJD) is the most common human disease caused by prions. Prions cause incurable neurologic diseases and are resistant to common disinfection methods. In our field, reusable washable endoscopes are used in endoscopic procedures where they may be exposed to at-risk tissues. We recognize the necessity to develop a framework for handling and disposal of endoscopes used in suspected or confirmed cases of CJD or prion disease, mitigating the risk of iatrogenic transmission.
Methods: With our institutional infection control and infectious diseases departments, we established a comprehensive protocol for handling and disposing of endoscopes at Mayo Clinic, in the instance of a suspected or confirmed case of CJD/prion disease. This process is dictated by the critical level of endoscopes and tissue risk level.
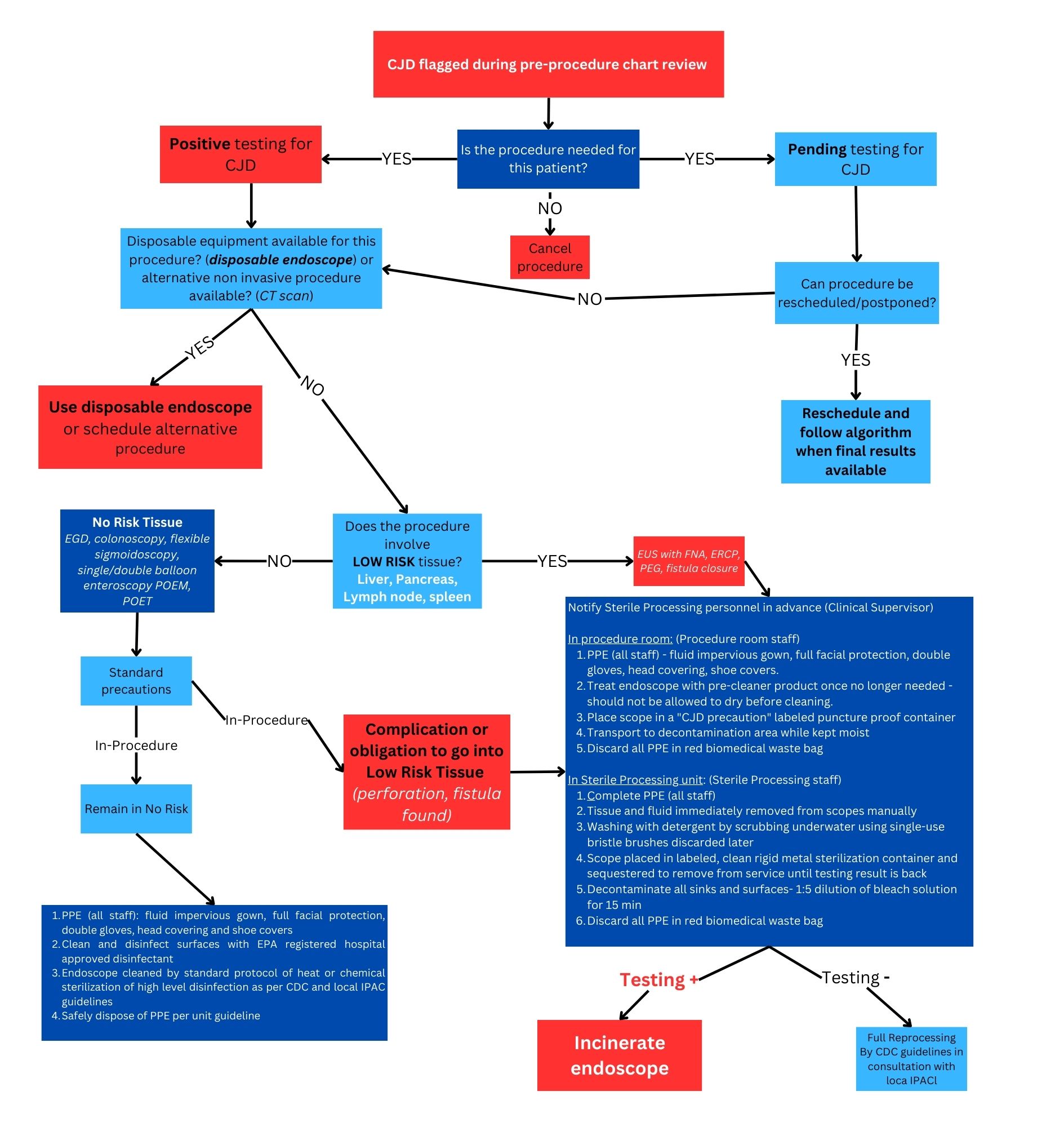
Results: Our protocol is based on endoscope classification according to the Spaulding Criteria and the risk level of tissues encountered in endoscopy. Endoscopes come in contact with mucous membranes meaning they are Semi-critical medical items. High risk tissues are neurological tissues not seen in endoscopy. Low risk tissues are involved in procedures such as endoscopic ultrasound with fine needle aspiration (lymph node, pancreas), and endoscopic retrograde cholangiopancreatography (liver and liver channels, pancreas). No-risk ones include the saliva, intestines, whole blood, encountered in any procedure (esophagogastroduodenoscopy, colonoscopy). Endoscopes exposed to no-risk tissue require standard instrument reprocessing by sterilization or high-level disinfection. In anticipated exposure to low-risk tissue, the use of disposable endoscopes is advised. They are disposed of by incineration at the end of the procedure. If disposable instruments are not available, reusable ones undergo a unique cleaning process while kept moist. Then, they are sequestered in a sterilization container. If the result of CJD/prion investigation is positive, endoscopes are incinerated. If testing is negative, they are reprocessed by standard methods.
Discussion: The nature of CJD or prion disease poses a burden on infection control in healthcare settings, notably endoscopic procedures. Reusable endoscopes carry a virtual risk of iatrogenic transmission. We propose a multi-disciplinary, best-evidence approach to handling endoscopy equipment for patients with suspected or confirmed prion disease.
Disclosures:
Yara Salameh, MD1, Lea Sayegh, MD1, Michelle Meyer, PhD2, Miranda Hamlin, MA2, Manik Aggarwal, MBBS2, Barham Abu Dayyeh, MD2, Nayantara Coelho-Prabhu, MD, FACG2, Vinay Chandrasekhara, MD2, Amanda M. Johnson, MD2, Ryan Law, DO2, John Martin, MD2, Bret Petersen, MD2, Andrew Storm, MD2. P2387 - A Novel Protocol for Endoscope Handling and Disposal in the Setting of Creutzfeldt-Jakob and Other Prion Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Mayo Foundation for Medical Education and Research, Rochester, MN; 2Mayo Clinic, Rochester, MN
Introduction: Known as “slow virus” disease, Creutzfeldt-Jakob Disease (CJD) is the most common human disease caused by prions. Prions cause incurable neurologic diseases and are resistant to common disinfection methods. In our field, reusable washable endoscopes are used in endoscopic procedures where they may be exposed to at-risk tissues. We recognize the necessity to develop a framework for handling and disposal of endoscopes used in suspected or confirmed cases of CJD or prion disease, mitigating the risk of iatrogenic transmission.
Methods: With our institutional infection control and infectious diseases departments, we established a comprehensive protocol for handling and disposing of endoscopes at Mayo Clinic, in the instance of a suspected or confirmed case of CJD/prion disease. This process is dictated by the critical level of endoscopes and tissue risk level.
Results: Our protocol is based on endoscope classification according to the Spaulding Criteria and the risk level of tissues encountered in endoscopy. Endoscopes come in contact with mucous membranes meaning they are Semi-critical medical items. High risk tissues are neurological tissues not seen in endoscopy. Low risk tissues are involved in procedures such as endoscopic ultrasound with fine needle aspiration (lymph node, pancreas), and endoscopic retrograde cholangiopancreatography (liver and liver channels, pancreas). No-risk ones include the saliva, intestines, whole blood, encountered in any procedure (esophagogastroduodenoscopy, colonoscopy). Endoscopes exposed to no-risk tissue require standard instrument reprocessing by sterilization or high-level disinfection. In anticipated exposure to low-risk tissue, the use of disposable endoscopes is advised. They are disposed of by incineration at the end of the procedure. If disposable instruments are not available, reusable ones undergo a unique cleaning process while kept moist. Then, they are sequestered in a sterilization container. If the result of CJD/prion investigation is positive, endoscopes are incinerated. If testing is negative, they are reprocessed by standard methods.
Discussion: The nature of CJD or prion disease poses a burden on infection control in healthcare settings, notably endoscopic procedures. Reusable endoscopes carry a virtual risk of iatrogenic transmission. We propose a multi-disciplinary, best-evidence approach to handling endoscopy equipment for patients with suspected or confirmed prion disease.

Figure: Proposed protocol for endoscope handling and disposal in the setting of CJD or prion disease
CJD= Creutzfeldt-Jakob Disease
EGD= Esophagogastroduodenoscopy
POEM/POET= Per-oral endoscopic myotomy
EUS= Endoscopic ultrasound
FNA= Fine Needle Aspiration
PEG= Percutaneous Endoscopic Gastrostomy
ERCP= Endoscopic Retrograde Cholangiopancreatography
PPE= Personal Protective Equipment
EPA= Environmental Protection Agency
IPAC= Infection Prevention and Control
CJD= Creutzfeldt-Jakob Disease
EGD= Esophagogastroduodenoscopy
POEM/POET= Per-oral endoscopic myotomy
EUS= Endoscopic ultrasound
FNA= Fine Needle Aspiration
PEG= Percutaneous Endoscopic Gastrostomy
ERCP= Endoscopic Retrograde Cholangiopancreatography
PPE= Personal Protective Equipment
EPA= Environmental Protection Agency
IPAC= Infection Prevention and Control
Disclosures:
Yara Salameh indicated no relevant financial relationships.
Lea Sayegh indicated no relevant financial relationships.
Michelle Meyer indicated no relevant financial relationships.
Miranda Hamlin indicated no relevant financial relationships.
Manik Aggarwal indicated no relevant financial relationships.
Barham Abu Dayyeh indicated no relevant financial relationships.
Nayantara Coelho-Prabhu: Iterative Health – Advisory Committee/Board Member.
Vinay Chandrasekhara indicated no relevant financial relationships.
Amanda Johnson indicated no relevant financial relationships.
Ryan Law: boston scientific – Consultant, Grant/Research Support. Olympus – Consultant, Grant/Research Support.
John Martin indicated no relevant financial relationships.
Bret Petersen indicated no relevant financial relationships.
Andrew Storm: Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Enterasense – Grant/Research Support. Intuitive Surgical – Consultant. Olympus – Consultant. SofTac – Grant/Research Support.
Yara Salameh, MD1, Lea Sayegh, MD1, Michelle Meyer, PhD2, Miranda Hamlin, MA2, Manik Aggarwal, MBBS2, Barham Abu Dayyeh, MD2, Nayantara Coelho-Prabhu, MD, FACG2, Vinay Chandrasekhara, MD2, Amanda M. Johnson, MD2, Ryan Law, DO2, John Martin, MD2, Bret Petersen, MD2, Andrew Storm, MD2. P2387 - A Novel Protocol for Endoscope Handling and Disposal in the Setting of Creutzfeldt-Jakob and Other Prion Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
