Monday Poster Session
Category: General Endoscopy
P2391 - Time and Experience Do Not Lead to Improved Adenoma Detection Rate With Artificial Intelligence-Assisted Colonoscopy: An 11-Month Implementation Trial
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Tessa Herman, MD
University of Minnesota and Minneapolis VA Health Care System
Minneapolis, MN
Presenting Author(s)
Tessa Herman, MD1, Kawthar Mohamed, MD2, Daniela Guerrero Vinsard, MD1, Morgan Freeman, MD2, Amy Gravely, MA3, Anders Westanmo, PharmD, MBA3, Lisa Smith, RN3, Mohammad Bilal, MD1, Brian Hanson, MD3
1University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN; 2University of Minnesota, Minneapolis, MN; 3Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Artificial intelligence-assisted colonoscopy (AIAC) with computer-aided detection (CADe) technology is designed to improve colonoscopy quality. While randomized controlled trial data is largely positive with improved adenoma detection rate (ADR) using AIAC, real-world implementation trials demonstrate mixed results, including a six-month pre-post implementation trial we previously reported on using two AIAC technologies which showed lower ADR with AIAC. However, current implementation trials are limited by short study durations and thus the impact of AIAC on ADR over time is unknown.
Methods: We extended the duration of our single-center, quasi-experimental implementation study to 11 months using one AIAC technology with the aim to evaluate if the ADR improves over time with continued AIAC use and experience. We compared the ADR at 6-month intervals after AIAC implementation to each other, as well as to a historical cohort with unassisted colonoscopy. Colonoscopies performed for screening, surveillance, or positive fecal immunochemical test indications were included. Secondary endpoints included sessile serrated lesion (SSL), hyperplastic polyp, and benign, non-adenomatous, non-hyperplastic (BNANH) lesion (e.g. lymphoid aggregate, normal mucosa) detection rates. Statistical analysis was performed using Pearson’s chi-square or ANOVA Type III F-test, as applicable.
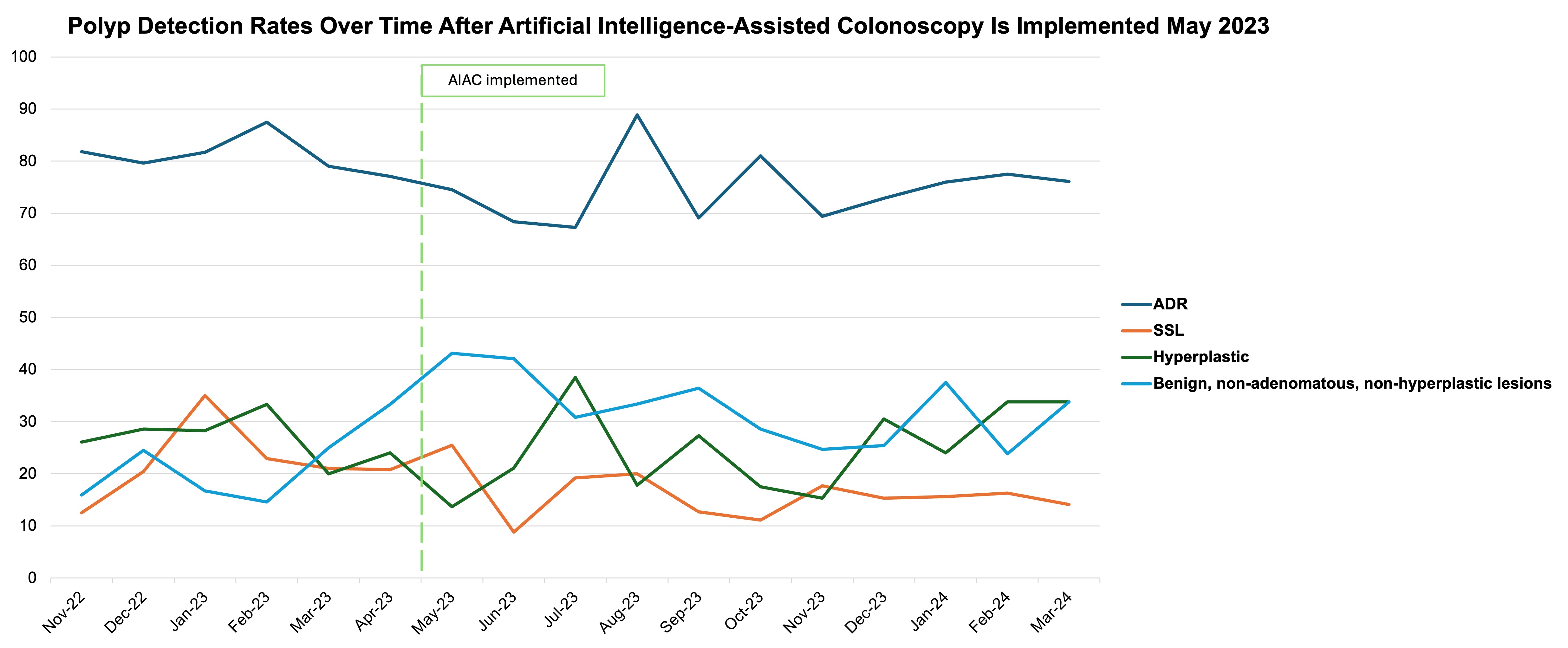
Results: We evaluated 295 AIAC colonoscopies in the first 6-month interval (May-October 2023) and 419 AIAC colonoscopies in the second 5-month interval (November 2023-March 2024). Groups were balanced without differences in patient demographics (age, gender, body mass index), endoscopists involved, or polyps per colonoscopy. ADR did not improve between AIAC cohorts (74.2% versus 74.7%, p=0.89). Similarly, we did not see improvement in other polyp detection rates with continued AIAC use (Table 1), although there was a near-significant trend towards decreased resection of BNANH lesions (36.3% versus 29.4%, p=0.05) and rebounding ADR over time (Figure 1).
Discussion: Our real-world implementation study failed to show improvement in ADR with continued use and experience with AIAC at nearly 1 year after its implementation. This is unexpected as we hypothesized that with time and experience ADR would improve. This finding suggests a deficit of the CADe technology in detecting adenomatous lesions in our cohort and that time and experience do not enhance the endoscopist-AI interaction.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Tessa Herman, MD1, Kawthar Mohamed, MD2, Daniela Guerrero Vinsard, MD1, Morgan Freeman, MD2, Amy Gravely, MA3, Anders Westanmo, PharmD, MBA3, Lisa Smith, RN3, Mohammad Bilal, MD1, Brian Hanson, MD3. P2391 - Time and Experience Do Not Lead to Improved Adenoma Detection Rate With Artificial Intelligence-Assisted Colonoscopy: An 11-Month Implementation Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN; 2University of Minnesota, Minneapolis, MN; 3Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Artificial intelligence-assisted colonoscopy (AIAC) with computer-aided detection (CADe) technology is designed to improve colonoscopy quality. While randomized controlled trial data is largely positive with improved adenoma detection rate (ADR) using AIAC, real-world implementation trials demonstrate mixed results, including a six-month pre-post implementation trial we previously reported on using two AIAC technologies which showed lower ADR with AIAC. However, current implementation trials are limited by short study durations and thus the impact of AIAC on ADR over time is unknown.
Methods: We extended the duration of our single-center, quasi-experimental implementation study to 11 months using one AIAC technology with the aim to evaluate if the ADR improves over time with continued AIAC use and experience. We compared the ADR at 6-month intervals after AIAC implementation to each other, as well as to a historical cohort with unassisted colonoscopy. Colonoscopies performed for screening, surveillance, or positive fecal immunochemical test indications were included. Secondary endpoints included sessile serrated lesion (SSL), hyperplastic polyp, and benign, non-adenomatous, non-hyperplastic (BNANH) lesion (e.g. lymphoid aggregate, normal mucosa) detection rates. Statistical analysis was performed using Pearson’s chi-square or ANOVA Type III F-test, as applicable.
Results: We evaluated 295 AIAC colonoscopies in the first 6-month interval (May-October 2023) and 419 AIAC colonoscopies in the second 5-month interval (November 2023-March 2024). Groups were balanced without differences in patient demographics (age, gender, body mass index), endoscopists involved, or polyps per colonoscopy. ADR did not improve between AIAC cohorts (74.2% versus 74.7%, p=0.89). Similarly, we did not see improvement in other polyp detection rates with continued AIAC use (Table 1), although there was a near-significant trend towards decreased resection of BNANH lesions (36.3% versus 29.4%, p=0.05) and rebounding ADR over time (Figure 1).
Discussion: Our real-world implementation study failed to show improvement in ADR with continued use and experience with AIAC at nearly 1 year after its implementation. This is unexpected as we hypothesized that with time and experience ADR would improve. This finding suggests a deficit of the CADe technology in detecting adenomatous lesions in our cohort and that time and experience do not enhance the endoscopist-AI interaction.

Figure: Figure 1: Adenoma and benign, non-adenomatous, non-hyperplastic lesion detection rates over time – Evaluating adenoma detection rate (ADR) and benign, non-adenomatous, non-hyperplastic (BNANH) lesion detection rate on a month-to-month basis revealed a potential rebound in improving ADR over time, as well as overall decreased BNANH resection rates.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Tessa Herman indicated no relevant financial relationships.
Kawthar Mohamed indicated no relevant financial relationships.
Daniela Guerrero Vinsard indicated no relevant financial relationships.
Morgan Freeman indicated no relevant financial relationships.
Amy Gravely indicated no relevant financial relationships.
Anders Westanmo indicated no relevant financial relationships.
Lisa Smith indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Speakers Bureau.
Brian Hanson: Motus GI – Consultant.
Tessa Herman, MD1, Kawthar Mohamed, MD2, Daniela Guerrero Vinsard, MD1, Morgan Freeman, MD2, Amy Gravely, MA3, Anders Westanmo, PharmD, MBA3, Lisa Smith, RN3, Mohammad Bilal, MD1, Brian Hanson, MD3. P2391 - Time and Experience Do Not Lead to Improved Adenoma Detection Rate With Artificial Intelligence-Assisted Colonoscopy: An 11-Month Implementation Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
