Monday Poster Session
Category: Colorectal Cancer Prevention
P2147 - Projected Benefit and Cost-Effectiveness of Offering a Choice of Blood-Based Screening Versus Existing Screening Tests for Colorectal Cancer in Two Real-World Settings
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Reinier G.S Meester, MS, PhD
Freenome, Inc.
South San Francisco, CA
Presenting Author(s)
Reinier G.S.. Meester, MS, PhD1, Andrew J.. Piscitello, 1, Lance Baldo, MD2, Noelle M. Griffin, PhD2, Peter S.. Liang, MD, MPH3
1Freenome, Inc., South San Francisco, CA; 2Freenome, South San Francisco, CA; 3NYU Langone Health, New York, NY
Introduction: Emerging blood tests for colorectal cancer (CRC) may improve screening outcomes. However, there is concern that replacing existing tests with blood tests could adversely impact health outcomes and costs due to lower sensitivity for advanced precursors of current blood tests. Two recent studies in patients who previously declined screening, found that offering a choice of blood testing vs. existing tests increased overall adherence without substantial test replacement. We estimate the long-term clinical and economic impact of these observed patterns.
Methods: An established CISNET model was replicated, validated, and then used to evaluate the observed mix of screening across study control and intervention arms. Assumptions utilized registry-, clinical- and payment data. Assumed adherence to colonoscopy every 10 years, annual fecal immunochemical testing (FIT) and triennial blood testing was as observed in Liang et al. and Coronado et al.1,2 For blood testing, assumed performance (74% CRC sensitivity, 90% specificity, 10% advanced precancerous lesion sensitivity) satisfied minimum U.S. coverage criteria, and assumed costs matched sDNA-FIT’s. Outcomes included deaths averted, life-years gained (LYG), and incremental cost/LYG for offering the choice of blood testing.
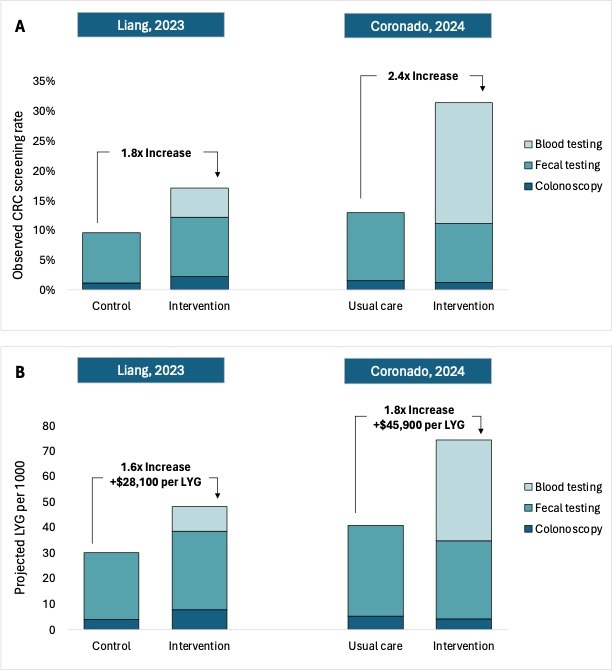
Results: In Liang et al., screening comprised 2.2% colonoscopy, 9.9% FIT and 5.0% blood testing in the intervention arm, vs. 1.1% colonoscopy and 8.4% FIT in the control arm (Figure, A). With these adherence rates, offering blood testing in addition to existing tests was projected to result in 1.6 additional CRC deaths averted and 18.2 LYG per 1000 persons at a cost of $28,100/LYG (Figure, B). In Coronado et al., screening comprised 1.2% colonoscopy screening, 9.9% FIT, and 20.4% blood testing in the intervention arm, vs. 1.5% colonoscopy screening and 11.5% FIT in the usual care arm (Figure, A). Here, offering blood-based screening resulted in 2.9 projected CRC deaths averted and 33.6 LYG per 1000 persons at a cost of $45,900/LYG (Figure, B).
Discussion: Trial data and modeling suggest that offering patients a choice of blood-based CRC screening vs. existing tests may substantially improve outcomes at acceptable cost. More research is needed to understand the impact of first-line blood testing, longitudinal adherence, and incomplete colonoscopy follow-up.
Disclosures:
Reinier G.S.. Meester, MS, PhD1, Andrew J.. Piscitello, 1, Lance Baldo, MD2, Noelle M. Griffin, PhD2, Peter S.. Liang, MD, MPH3. P2147 - Projected Benefit and Cost-Effectiveness of Offering a Choice of Blood-Based Screening Versus Existing Screening Tests for Colorectal Cancer in Two Real-World Settings, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Freenome, Inc., South San Francisco, CA; 2Freenome, South San Francisco, CA; 3NYU Langone Health, New York, NY
Introduction: Emerging blood tests for colorectal cancer (CRC) may improve screening outcomes. However, there is concern that replacing existing tests with blood tests could adversely impact health outcomes and costs due to lower sensitivity for advanced precursors of current blood tests. Two recent studies in patients who previously declined screening, found that offering a choice of blood testing vs. existing tests increased overall adherence without substantial test replacement. We estimate the long-term clinical and economic impact of these observed patterns.
Methods: An established CISNET model was replicated, validated, and then used to evaluate the observed mix of screening across study control and intervention arms. Assumptions utilized registry-, clinical- and payment data. Assumed adherence to colonoscopy every 10 years, annual fecal immunochemical testing (FIT) and triennial blood testing was as observed in Liang et al. and Coronado et al.1,2 For blood testing, assumed performance (74% CRC sensitivity, 90% specificity, 10% advanced precancerous lesion sensitivity) satisfied minimum U.S. coverage criteria, and assumed costs matched sDNA-FIT’s. Outcomes included deaths averted, life-years gained (LYG), and incremental cost/LYG for offering the choice of blood testing.
Results: In Liang et al., screening comprised 2.2% colonoscopy, 9.9% FIT and 5.0% blood testing in the intervention arm, vs. 1.1% colonoscopy and 8.4% FIT in the control arm (Figure, A). With these adherence rates, offering blood testing in addition to existing tests was projected to result in 1.6 additional CRC deaths averted and 18.2 LYG per 1000 persons at a cost of $28,100/LYG (Figure, B). In Coronado et al., screening comprised 1.2% colonoscopy screening, 9.9% FIT, and 20.4% blood testing in the intervention arm, vs. 1.5% colonoscopy screening and 11.5% FIT in the usual care arm (Figure, A). Here, offering blood-based screening resulted in 2.9 projected CRC deaths averted and 33.6 LYG per 1000 persons at a cost of $45,900/LYG (Figure, B).
Discussion: Trial data and modeling suggest that offering patients a choice of blood-based CRC screening vs. existing tests may substantially improve outcomes at acceptable cost. More research is needed to understand the impact of first-line blood testing, longitudinal adherence, and incomplete colonoscopy follow-up.

Figure: Observed Adherence (A) and Projected Benefit and Cost-Effectiveness (B) of Offering a Choice of
Blood-Based Screening vs. Existing Tests for Colorectal Cancer.
Blood-Based Screening vs. Existing Tests for Colorectal Cancer.
Disclosures:
Reinier Meester: Freenome Holdings Inc. – Employee.
Andrew Piscitello: Caris Life Sciences – Consultant. ClearNote Health – Consultant. Elephas – Consultant. Freenome Holdings Inc. – Employee. Nucleix – Consultant. Thermo Fisher Scientific – Consultant.
Lance Baldo: Freenome – Employee, Stock Options.
Noelle Griffin: Freenome Holdings Inc – Employee. Pfizer Inc – Employee.
Peter Liang: Freenome – Grant/Research Support. Guardant Health – Advisory Committee/Board Member. Natera – Advisory Committee/Board Member.
Reinier G.S.. Meester, MS, PhD1, Andrew J.. Piscitello, 1, Lance Baldo, MD2, Noelle M. Griffin, PhD2, Peter S.. Liang, MD, MPH3. P2147 - Projected Benefit and Cost-Effectiveness of Offering a Choice of Blood-Based Screening Versus Existing Screening Tests for Colorectal Cancer in Two Real-World Settings, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
