Monday Poster Session
Category: Colon
P1938 - Assessing Enrollment Disparities by Demographics in Colorectal Cancer Clinical Trials Across the United States
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Wissam Ghusn, MD
Boston Medical Center
Boston, MA
Presenting Author(s)
Wissam Ghusn, MD1, Tamara Kadi, MD2, Johnny Bou Saba, MD3, Elisia Maalouf, MD4, Lynn Kobeissi, MD5, Marita Salame, MD6, Donna Maria Abboud, MD7, Marcel Yibirin, MD8, Rudy Mrad, MD4, Karim Al Annan, MD9, Maria A. Espinosa, MD4, Sandra Quezada, MD, MS10, Aline Charabaty, MD11
1Boston Medical Center, Boston, MA; 2University of Pittsburgh Medical Center, Pittsburgh, PA; 3University of Pittsburgh, Pittsburgh, PA; 4Mayo Clinic, Rochester, MN; 5American University of Beirut, Beirut, Beyrouth, Lebanon; 6Indiana University School of Medicine, Indianapolis, IN; 7Mayo Clinic, Miami, FL; 8Boston Medical Center, Boston University School of Medicine, Boston, MA; 9Mayo Clinic, Hartford, CT; 10University of Maryland School of Medicine, Baltimore, MD; 11Johns Hopkins University School of Medicine, Washington, DC
Introduction: Colorectal cancer (CRC) remains a leading cause of morbidity and mortality, with disparities in incidence, prevalence and mortality rates among different demographic and racial groups presenting a significant public health concern. This study aims to assess demographic representation in CRC clinical trials conducted in the United States and compare these findings to national epidemiological data.
Methods: We conducted a retrospective review of all clinical trials from ClinicalTrials.gov that included studies involving patients with CRC until March 2024. We had no restriction to specific study types (i.e., not restricted to therapeutic trial). To maintain a focus on the US population and to ensure the demographic relevance of our findings, we excluded trials that included international sites or participants from outside the US. The demographic variables of interest included age, sex, and race/ethnicity of participants, which were compared to national CRC incidence and mortality data from the demographic data reported by the American Cancer Society between 2020-2022.
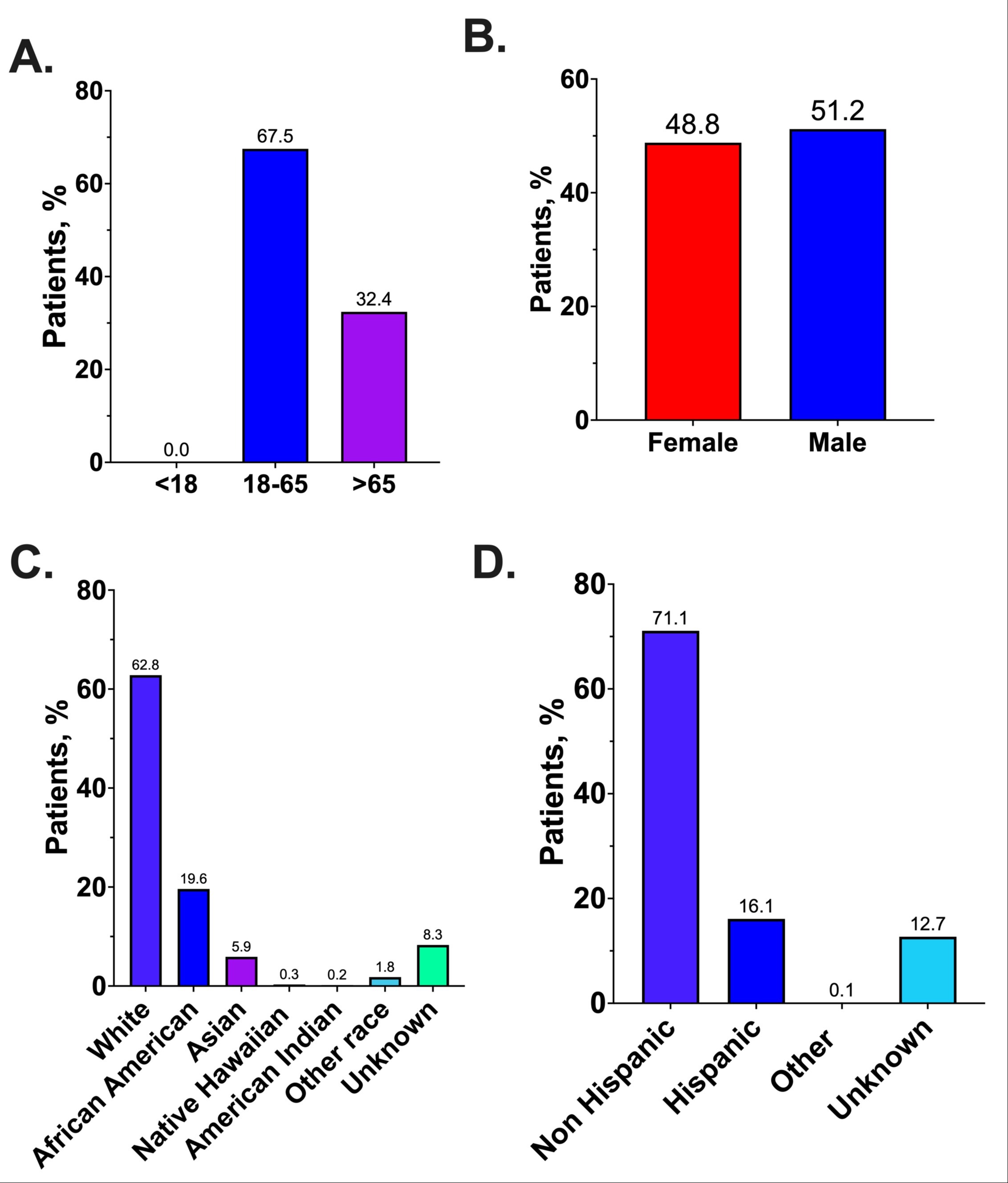
Results: A total of 345 trials were included involving 181,579 participants. The average age of participants was 55.9 years, with 67.5% aged between 18-65, and only 32.5% older than 65 (Table 1, Figure 1 A). This contrasts with the median age of CRC diagnosis at 66 years for men and 69 for women, suggesting underrepresentation of the elderly in clinical trials. Sex distribution in clinical trials showed a female participation rate of 48.8%, which does not fully reflect the sex-based incidence, with men having about a 30% higher rate of CRC (Figure 1B). Racial distribution within these trials revealed significant imbalances, with White participants constituting 62.8% of trial populations and African Americans 19.6%, despite African Americans bearing a disproportionately higher incidence of 20% and mortality of 40%, compared to White individuals (Figure 1C-D).
Discussion: Our study elucidates considerable disparities in the age and racial representation of CRC clinical trial participants when compared to the demographic patterns observed within the general population. These results are unique as they shed light not only on the racial/ethnic disparities, but also age disparities which is of utmost importance to address. It is critical to rectify these imbalances by implementing recruitment strategies that ensure diverse and representative participation in CRC clinical research.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Wissam Ghusn, MD1, Tamara Kadi, MD2, Johnny Bou Saba, MD3, Elisia Maalouf, MD4, Lynn Kobeissi, MD5, Marita Salame, MD6, Donna Maria Abboud, MD7, Marcel Yibirin, MD8, Rudy Mrad, MD4, Karim Al Annan, MD9, Maria A. Espinosa, MD4, Sandra Quezada, MD, MS10, Aline Charabaty, MD11. P1938 - Assessing Enrollment Disparities by Demographics in Colorectal Cancer Clinical Trials Across the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Boston Medical Center, Boston, MA; 2University of Pittsburgh Medical Center, Pittsburgh, PA; 3University of Pittsburgh, Pittsburgh, PA; 4Mayo Clinic, Rochester, MN; 5American University of Beirut, Beirut, Beyrouth, Lebanon; 6Indiana University School of Medicine, Indianapolis, IN; 7Mayo Clinic, Miami, FL; 8Boston Medical Center, Boston University School of Medicine, Boston, MA; 9Mayo Clinic, Hartford, CT; 10University of Maryland School of Medicine, Baltimore, MD; 11Johns Hopkins University School of Medicine, Washington, DC
Introduction: Colorectal cancer (CRC) remains a leading cause of morbidity and mortality, with disparities in incidence, prevalence and mortality rates among different demographic and racial groups presenting a significant public health concern. This study aims to assess demographic representation in CRC clinical trials conducted in the United States and compare these findings to national epidemiological data.
Methods: We conducted a retrospective review of all clinical trials from ClinicalTrials.gov that included studies involving patients with CRC until March 2024. We had no restriction to specific study types (i.e., not restricted to therapeutic trial). To maintain a focus on the US population and to ensure the demographic relevance of our findings, we excluded trials that included international sites or participants from outside the US. The demographic variables of interest included age, sex, and race/ethnicity of participants, which were compared to national CRC incidence and mortality data from the demographic data reported by the American Cancer Society between 2020-2022.
Results: A total of 345 trials were included involving 181,579 participants. The average age of participants was 55.9 years, with 67.5% aged between 18-65, and only 32.5% older than 65 (Table 1, Figure 1 A). This contrasts with the median age of CRC diagnosis at 66 years for men and 69 for women, suggesting underrepresentation of the elderly in clinical trials. Sex distribution in clinical trials showed a female participation rate of 48.8%, which does not fully reflect the sex-based incidence, with men having about a 30% higher rate of CRC (Figure 1B). Racial distribution within these trials revealed significant imbalances, with White participants constituting 62.8% of trial populations and African Americans 19.6%, despite African Americans bearing a disproportionately higher incidence of 20% and mortality of 40%, compared to White individuals (Figure 1C-D).
Discussion: Our study elucidates considerable disparities in the age and racial representation of CRC clinical trial participants when compared to the demographic patterns observed within the general population. These results are unique as they shed light not only on the racial/ethnic disparities, but also age disparities which is of utmost importance to address. It is critical to rectify these imbalances by implementing recruitment strategies that ensure diverse and representative participation in CRC clinical research.

Figure: Figure 1: Age (A), sex (B), race (C), and ethnic (D) distribution of enrolled participants in US clinical trials for CRC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Wissam Ghusn indicated no relevant financial relationships.
Tamara Kadi indicated no relevant financial relationships.
Johnny Bou Saba indicated no relevant financial relationships.
Elisia Maalouf indicated no relevant financial relationships.
Lynn Kobeissi indicated no relevant financial relationships.
Marita Salame indicated no relevant financial relationships.
Donna Maria Abboud indicated no relevant financial relationships.
Marcel Yibirin indicated no relevant financial relationships.
Rudy Mrad indicated no relevant financial relationships.
Karim Al Annan indicated no relevant financial relationships.
Maria A. Espinosa indicated no relevant financial relationships.
Sandra Quezada: Johnson and Johnson – Advisory Committee/Board Member, Consultant. Lilly – Advisory Committee/Board Member.
Aline Charabaty: AbbVie – Advisor or Review Panel Member, Consultant. Eli Lilly – Advisor or Review Panel Member, Consultant. Janssen – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Wissam Ghusn, MD1, Tamara Kadi, MD2, Johnny Bou Saba, MD3, Elisia Maalouf, MD4, Lynn Kobeissi, MD5, Marita Salame, MD6, Donna Maria Abboud, MD7, Marcel Yibirin, MD8, Rudy Mrad, MD4, Karim Al Annan, MD9, Maria A. Espinosa, MD4, Sandra Quezada, MD, MS10, Aline Charabaty, MD11. P1938 - Assessing Enrollment Disparities by Demographics in Colorectal Cancer Clinical Trials Across the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
