Monday Poster Session
Category: Biliary/Pancreas
P1780 - Safety and Tolerability of TH104, Buccal Nalmefene, in Patients With Cholestatic Liver Disease and Pruritus
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
- NB
Nir Barak, MD
Tharimmune Inc.
Bridgewater, NJ
Presenting Author(s)
Nir Barak, MD, Sireesj Appajosyula, PharmD
Tharimmune Inc., Bridgewater, NJ
Introduction: Opioid antagonists, such as nalmefene, are recomended by the AASLD as a third line therapy (off-label use) for pruritus in patients with cholestatic liver disease. Potential side effects, such as opioid withdrawal-like reactions, may be associated with this treatment. Buccal drug administration, bypass the liver first-path-metabolism, and therefore may be a better treatment option in patients with liver disease. We conducted a safety and tolerability study of buccal nalmefene, in patients with mild and moderate liver disease.
Methods: In this study, we used TH104, a proprietary formulation of nalmefene, 2 mg in strength. TH104 is a buccal film containing nalmefene, which has a relatively high absolute bioavailability and an improved PK profile, compared to IV and oral formulations. Patients with cholestatic liver disease and a known history of persistent generalized pruritus for at least 4 weeks prior to screening were included. After an overnight fasting of 10 hours, subjects received one dose of 2 mg of TH104. Apart of capturing adverse events (AEs), we used the Clinical Opiate Withdrawal Scale (COWS) to rate common signs and symptoms of opiate withdrawal. AEs were recorded pre dose and up to 7 days after dosing. COWS scores were captured pre-dosing and 2 hours after dosing (at Tmax of nalmefene). Fisher's exact test was used to analyze the data.
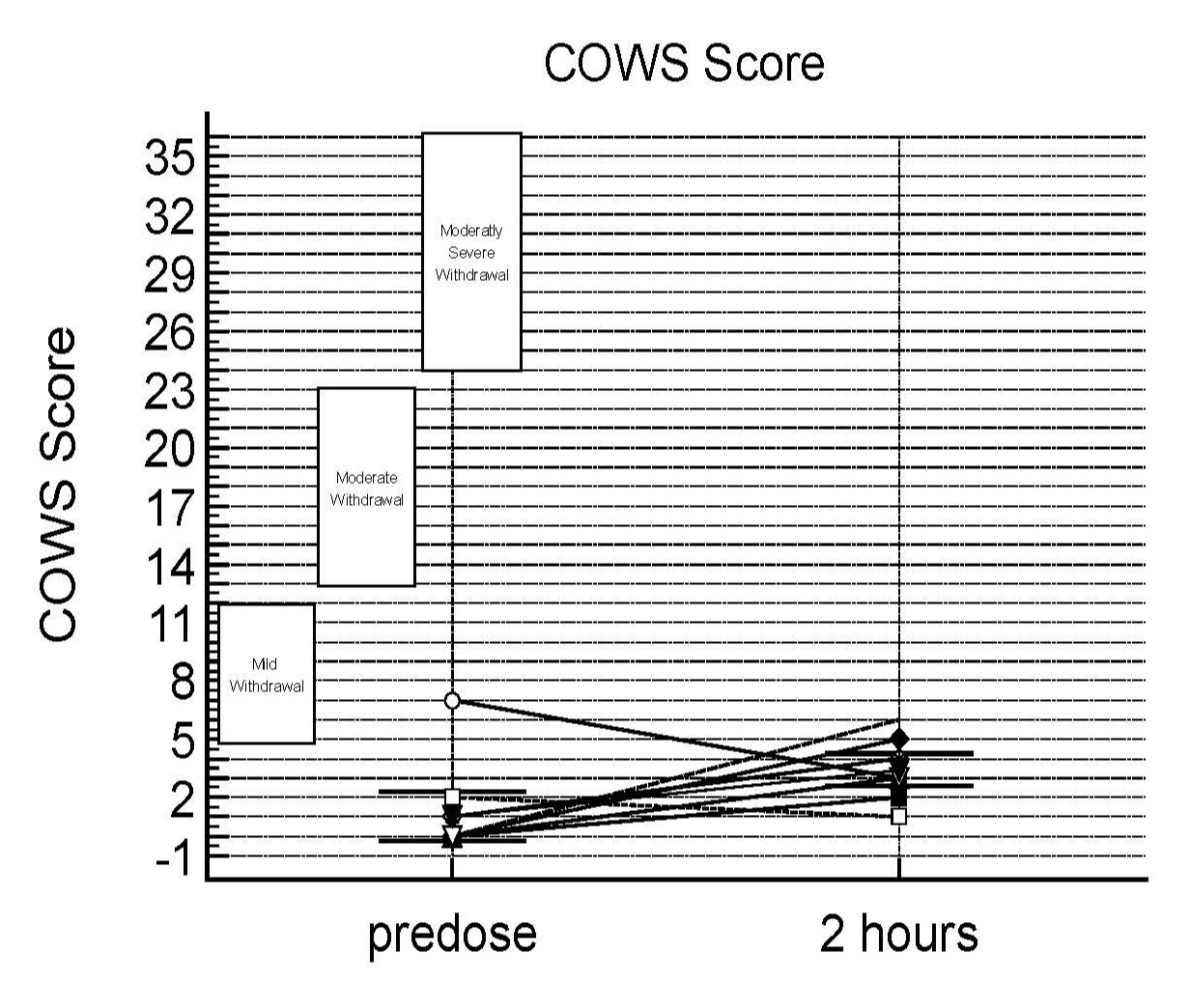
Results: Nineteen patients were screened, and twelve patients were enrolled in this study. Ten subjects were male, and two were female; six were Child-Pugh class A, and six were Child-Pugh class B. A total of 2 AEs (headache) were reported in 2 subjects over the course of the study. These AEs were mild and possibly related to study drug. There were no serious adverse events reported in the study. Two patients had an increase in COWS score to 5 and 6, and 1 patient experienced an improvement in COWS score that dropped from 7 to 3. These proportion changes were not statistically significant (p=0.20). Only 1 grade 3 COWS symptom was observed (Piloerection of hairs), and no grade 4 or 5 symptoms were recorded.
Discussion: The study results indicate that the use of TH104, 2 mg of buccal nalmefene in patients with mild and moderate cholestatic liver disease is safe and tolerable and not associated with opioid withdrawal syndrome.
Disclosures:
Nir Barak, MD, Sireesj Appajosyula, PharmD. P1780 - Safety and Tolerability of TH104, Buccal Nalmefene, in Patients With Cholestatic Liver Disease and Pruritus, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Tharimmune Inc., Bridgewater, NJ
Introduction: Opioid antagonists, such as nalmefene, are recomended by the AASLD as a third line therapy (off-label use) for pruritus in patients with cholestatic liver disease. Potential side effects, such as opioid withdrawal-like reactions, may be associated with this treatment. Buccal drug administration, bypass the liver first-path-metabolism, and therefore may be a better treatment option in patients with liver disease. We conducted a safety and tolerability study of buccal nalmefene, in patients with mild and moderate liver disease.
Methods: In this study, we used TH104, a proprietary formulation of nalmefene, 2 mg in strength. TH104 is a buccal film containing nalmefene, which has a relatively high absolute bioavailability and an improved PK profile, compared to IV and oral formulations. Patients with cholestatic liver disease and a known history of persistent generalized pruritus for at least 4 weeks prior to screening were included. After an overnight fasting of 10 hours, subjects received one dose of 2 mg of TH104. Apart of capturing adverse events (AEs), we used the Clinical Opiate Withdrawal Scale (COWS) to rate common signs and symptoms of opiate withdrawal. AEs were recorded pre dose and up to 7 days after dosing. COWS scores were captured pre-dosing and 2 hours after dosing (at Tmax of nalmefene). Fisher's exact test was used to analyze the data.
Results: Nineteen patients were screened, and twelve patients were enrolled in this study. Ten subjects were male, and two were female; six were Child-Pugh class A, and six were Child-Pugh class B. A total of 2 AEs (headache) were reported in 2 subjects over the course of the study. These AEs were mild and possibly related to study drug. There were no serious adverse events reported in the study. Two patients had an increase in COWS score to 5 and 6, and 1 patient experienced an improvement in COWS score that dropped from 7 to 3. These proportion changes were not statistically significant (p=0.20). Only 1 grade 3 COWS symptom was observed (Piloerection of hairs), and no grade 4 or 5 symptoms were recorded.
Discussion: The study results indicate that the use of TH104, 2 mg of buccal nalmefene in patients with mild and moderate cholestatic liver disease is safe and tolerable and not associated with opioid withdrawal syndrome.

Figure: COWS score at predose and 2 hours post dosing (at Tmax)
Disclosures:
Nir Barak: Tharimmune Inc – Consultant.
Sireesj Appajosyula: Tharimmune Inc – Employee.
Nir Barak, MD, Sireesj Appajosyula, PharmD. P1780 - Safety and Tolerability of TH104, Buccal Nalmefene, in Patients With Cholestatic Liver Disease and Pruritus, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
