Sunday Poster Session
Category: Liver
P1288 - Labetalol and the Liver: A Rare Postpartum Encounter With Drug-Induced Liver Injury
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Akira Folk, DO
University of Arizona College of Medicine
Tucson, AZ
Presenting Author(s)
Brandon Witten, MD1, Akira Folk, DO1, Samuel H. Cheong, DO2, Malini Chauhan, MD1, Shivanand Bomman, MD1, Alexis Elliott, MD1, Haripriya Maddur, MD1
1University of Arizona College of Medicine, Tucson, AZ; 2Banner - University of Arizona, Tucson, AZ
Introduction: Labetalol is commonly prescribed for hypertension during pregnancy and the postpartum period due to its favorable safety profile. While it is generally well-tolerated, rare cases of drug-induced liver injury (DILI) associated with labetalol have been reported in the literature. Here, we describe a case of postpartum DILI attributed to labetalol.
Case Description/Methods: 37-year-old woman approximately 2 months post-partum presented to the ED with two days of diarrhea and progressive scleral icterus. Initial labs were significant for AST: 1235, ALT: 1176, Alkaline phosphatase: 237. Total bilirubin was also elevated to 16.6 with direct bilirubin at 12, INR: 1.3. Patient denied intravenous drug use, excessive alcohol consumption or taking medications/supplements aside from labetalol and insulin. Labetalol had been started in March 2023 when she was approximately 4 weeks pregnant.
Subsequently she was admitted to inpatient with hepatology consultation and started on N-Acetylcysteine. AMA, ANA, ASMA, HSV PCR, hepatitis A, B, C IgM/IgG and hepatitis E serologies were negative. MR abdomen was significant only for acute hepatitis.
Due to persistent elevated liver function tests liver biopsy was undertaken. Histology was significant for acute hepatitis with approximately 40% of hepatocyte necrosis/dropout. Additionally mixed periportal and lobular inflammatory infiltrate were present specifically lymphocytes, neutrophils, eosinophils with rare plasma cells. Finally no significant fibrosis/steatosis was identified.
Due to the inflammatory infiltrate present on histology she was started on Solu-Medrol 250 mg for 3 days eventually transitioning to oral steroids. The patient had a dramatic response to steroid therapy with LFTs decreasing by 50% in 48 hours AST: 1353 to 256 and ALT: 1305 to 668. Subsequently was discharged with outpatient hepatology follow-up and 3 months of oral prednisone with normalization in liver tests and total bilirubin AST: 15, ALT: 18, Tbili: 1.
Discussion: Few instances of labetalol induced DILI have been reported in the literature, our case is one of the first to describe it in the post-partum period. While steroid therapy is generally reserved for drug induced autoimmune hepatitis (DIAIH), this patient demonstrated significant improvement despite the lack of classic features of autoimmune hepatitis. Histologically the lack of plasma cells, presence of interface hepatitis suggested classic DILI and not DIAIH. Negative autoimmune serologies further supported this diagnosis.
Disclosures:
Brandon Witten, MD1, Akira Folk, DO1, Samuel H. Cheong, DO2, Malini Chauhan, MD1, Shivanand Bomman, MD1, Alexis Elliott, MD1, Haripriya Maddur, MD1. P1288 - Labetalol and the Liver: A Rare Postpartum Encounter With Drug-Induced Liver Injury, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Arizona College of Medicine, Tucson, AZ; 2Banner - University of Arizona, Tucson, AZ
Introduction: Labetalol is commonly prescribed for hypertension during pregnancy and the postpartum period due to its favorable safety profile. While it is generally well-tolerated, rare cases of drug-induced liver injury (DILI) associated with labetalol have been reported in the literature. Here, we describe a case of postpartum DILI attributed to labetalol.
Case Description/Methods: 37-year-old woman approximately 2 months post-partum presented to the ED with two days of diarrhea and progressive scleral icterus. Initial labs were significant for AST: 1235, ALT: 1176, Alkaline phosphatase: 237. Total bilirubin was also elevated to 16.6 with direct bilirubin at 12, INR: 1.3. Patient denied intravenous drug use, excessive alcohol consumption or taking medications/supplements aside from labetalol and insulin. Labetalol had been started in March 2023 when she was approximately 4 weeks pregnant.
Subsequently she was admitted to inpatient with hepatology consultation and started on N-Acetylcysteine. AMA, ANA, ASMA, HSV PCR, hepatitis A, B, C IgM/IgG and hepatitis E serologies were negative. MR abdomen was significant only for acute hepatitis.
Due to persistent elevated liver function tests liver biopsy was undertaken. Histology was significant for acute hepatitis with approximately 40% of hepatocyte necrosis/dropout. Additionally mixed periportal and lobular inflammatory infiltrate were present specifically lymphocytes, neutrophils, eosinophils with rare plasma cells. Finally no significant fibrosis/steatosis was identified.
Due to the inflammatory infiltrate present on histology she was started on Solu-Medrol 250 mg for 3 days eventually transitioning to oral steroids. The patient had a dramatic response to steroid therapy with LFTs decreasing by 50% in 48 hours AST: 1353 to 256 and ALT: 1305 to 668. Subsequently was discharged with outpatient hepatology follow-up and 3 months of oral prednisone with normalization in liver tests and total bilirubin AST: 15, ALT: 18, Tbili: 1.
Discussion: Few instances of labetalol induced DILI have been reported in the literature, our case is one of the first to describe it in the post-partum period. While steroid therapy is generally reserved for drug induced autoimmune hepatitis (DIAIH), this patient demonstrated significant improvement despite the lack of classic features of autoimmune hepatitis. Histologically the lack of plasma cells, presence of interface hepatitis suggested classic DILI and not DIAIH. Negative autoimmune serologies further supported this diagnosis.

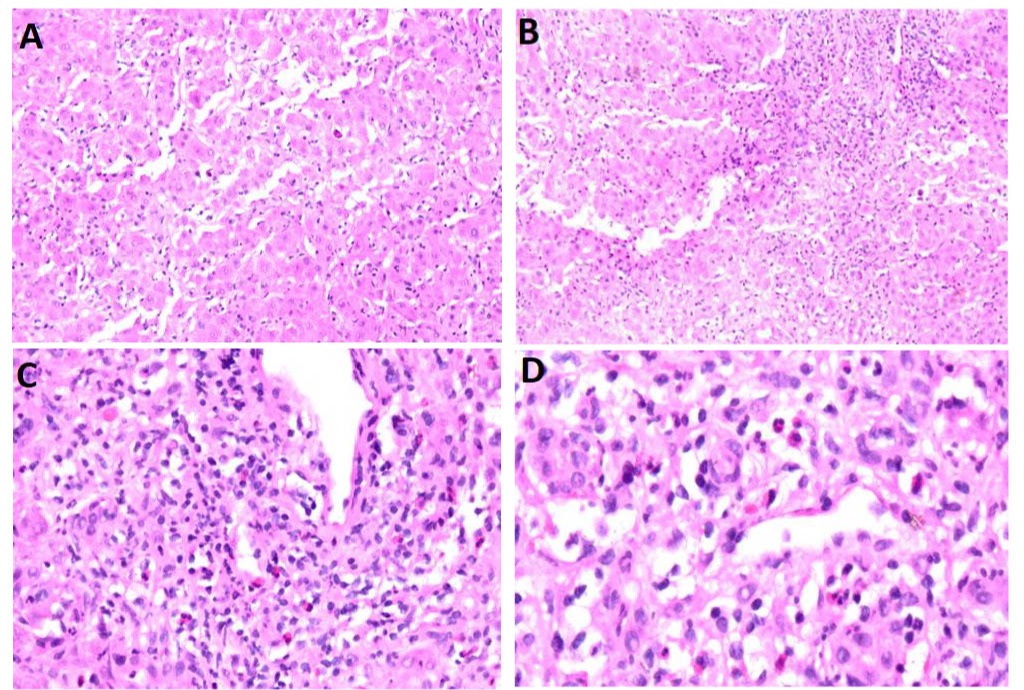
Figure: A: Acute hepatitis featuring apoptotic bodies
B: Hepatocyte drop out, consistent with severe acute hepatitis
C: Periportal inflammation with eosinophils, neutrophils and paucity of plasma cells suggesting classic DILI
D: Increased magnification of periportal inflammation with eosinophils, neutrophils and paucity of plasma cells suggesting classic DILI
B: Hepatocyte drop out, consistent with severe acute hepatitis
C: Periportal inflammation with eosinophils, neutrophils and paucity of plasma cells suggesting classic DILI
D: Increased magnification of periportal inflammation with eosinophils, neutrophils and paucity of plasma cells suggesting classic DILI
Disclosures:
Brandon Witten indicated no relevant financial relationships.
Akira Folk indicated no relevant financial relationships.
Samuel Cheong indicated no relevant financial relationships.
Malini Chauhan indicated no relevant financial relationships.
Shivanand Bomman indicated no relevant financial relationships.
Alexis Elliott indicated no relevant financial relationships.
Haripriya Maddur indicated no relevant financial relationships.
Brandon Witten, MD1, Akira Folk, DO1, Samuel H. Cheong, DO2, Malini Chauhan, MD1, Shivanand Bomman, MD1, Alexis Elliott, MD1, Haripriya Maddur, MD1. P1288 - Labetalol and the Liver: A Rare Postpartum Encounter With Drug-Induced Liver Injury, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
