Sunday Poster Session
Category: IBD
P0913 - How Many Doses of Infliximab Should Be Used in Immune Checkpoint Inhibitor-Induced Colitis? – A Retrospective Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Rupayan Kundu, MD
Cleveland Clinic Foundation
Cleveland, OH
Presenting Author(s)
Rupayan Kundu, MD1, Arjun Chatterjee, MD1, Emily Zabor, 1, James Isaacs, MD1, Thach-Giao Truong, MD1, Joseph Sleiman, MD2, Pauline Funchain, MD3, Jessica Philpott, MD, PhD1, Lucy Kennedy, MD1
1Cleveland Clinic Foundation, Cleveland, OH; 2University of Pittsburgh Medical Center, Pittsburgh, PA; 3Stanford University School of Medicine, Stanford, CA
Introduction: Immune checkpoint inhibitor (ICI)-related colitis (ir-colitis) is a common immune-related adverse event (irAE). For patients with steroid-refractory ir-colitis, anti-tumor necrosis factor agents such as infliximab are recommended. However, clinicians face significant challenges in determining the optimal number of infliximab doses needed to manage ir-colitis while maintaining anti-tumor efficacy of ICI. It is not known whether administering maintenance infliximab concurrently with ICI worsens cancer outcomes. In this study, we investigated cancer progression in pts who received > 3 doses of infliximab for steroid-refractory ir colitis compared to those who received ≤3 doses.
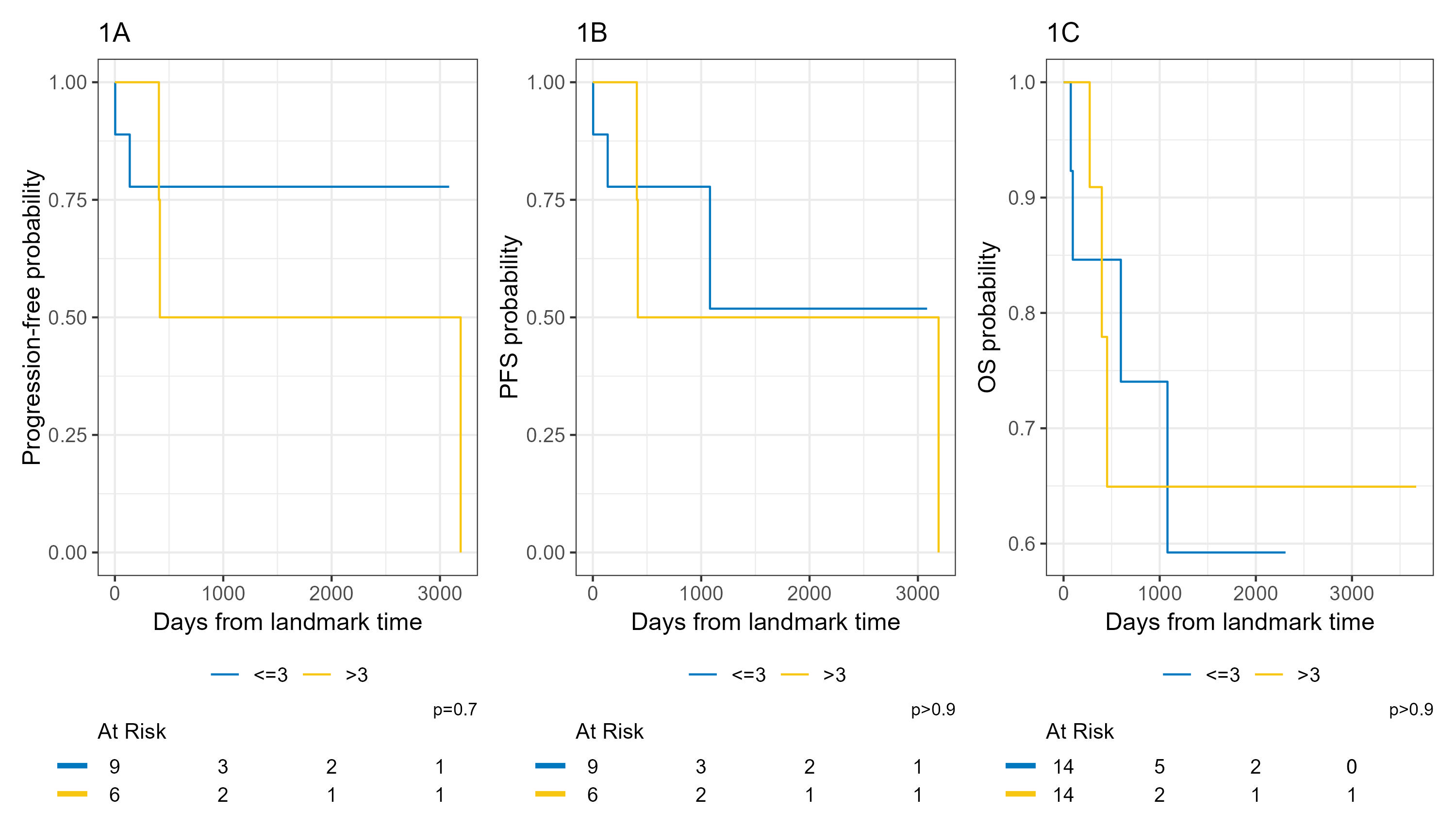
Methods: In this retrospective, single-center study, patients were categorized into 2 groups based on number of infliximab doses received: ≤3 doses and >3 doses. We calculated time to progression (TTP) (figure 1A), overall survival (OS) (figure 1B), and progression-free survival (PFS) (figure 1C) using a landmark analysis. The first three induction doses of infliximab were administered at 0, 2, and 6 weeks, followed by maintenance doses every 8 weeks. Consequently, we used a landmark time of 14 weeks for this analysis. Patients who had progressive disease at the onset of infliximab treatment were excluded from the TTP and PFS analyses.
Results: Of 29 pts, majority were white males with melanoma, and stage IV disease [Table 1]. 48.3% (n=14) of pts received >3 doses of infliximab with a median number of 6 doses (IQR: 4-7) [Table 1]. The median follow-up time among those without cancer progression was 703.5 days (IQR: 529.5 - 1190.5), still alive was 709 days (IQR: 371 – 1204), and still alive without progression, was 676 days (IQR: 481 - 1202). There were no significant differences in age, sex, ethnicity, cancer type, or cancer stage between the two groups. No significant difference in TTP, PFS, or OS were observed according to the number of infliximab cycles in the landmark analysis. [Figure 1]
Discussion: There was no association between receipt of > 3 doses of infliximab and worsened cancer outcomes, including PFS and OS, in our series, failing to detect cancer risk with longer term infliximab therapy. However, given the limitations of our study, including small sample size, single-center analysis, retrospective design, and lack of diversity, further prospective studies are warranted to confirm these findings and optimize infliximab dosing strategies.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Rupayan Kundu, MD1, Arjun Chatterjee, MD1, Emily Zabor, 1, James Isaacs, MD1, Thach-Giao Truong, MD1, Joseph Sleiman, MD2, Pauline Funchain, MD3, Jessica Philpott, MD, PhD1, Lucy Kennedy, MD1. P0913 - How Many Doses of Infliximab Should Be Used in Immune Checkpoint Inhibitor-Induced Colitis? – A Retrospective Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Cleveland Clinic Foundation, Cleveland, OH; 2University of Pittsburgh Medical Center, Pittsburgh, PA; 3Stanford University School of Medicine, Stanford, CA
Introduction: Immune checkpoint inhibitor (ICI)-related colitis (ir-colitis) is a common immune-related adverse event (irAE). For patients with steroid-refractory ir-colitis, anti-tumor necrosis factor agents such as infliximab are recommended. However, clinicians face significant challenges in determining the optimal number of infliximab doses needed to manage ir-colitis while maintaining anti-tumor efficacy of ICI. It is not known whether administering maintenance infliximab concurrently with ICI worsens cancer outcomes. In this study, we investigated cancer progression in pts who received > 3 doses of infliximab for steroid-refractory ir colitis compared to those who received ≤3 doses.
Methods: In this retrospective, single-center study, patients were categorized into 2 groups based on number of infliximab doses received: ≤3 doses and >3 doses. We calculated time to progression (TTP) (figure 1A), overall survival (OS) (figure 1B), and progression-free survival (PFS) (figure 1C) using a landmark analysis. The first three induction doses of infliximab were administered at 0, 2, and 6 weeks, followed by maintenance doses every 8 weeks. Consequently, we used a landmark time of 14 weeks for this analysis. Patients who had progressive disease at the onset of infliximab treatment were excluded from the TTP and PFS analyses.
Results: Of 29 pts, majority were white males with melanoma, and stage IV disease [Table 1]. 48.3% (n=14) of pts received >3 doses of infliximab with a median number of 6 doses (IQR: 4-7) [Table 1]. The median follow-up time among those without cancer progression was 703.5 days (IQR: 529.5 - 1190.5), still alive was 709 days (IQR: 371 – 1204), and still alive without progression, was 676 days (IQR: 481 - 1202). There were no significant differences in age, sex, ethnicity, cancer type, or cancer stage between the two groups. No significant difference in TTP, PFS, or OS were observed according to the number of infliximab cycles in the landmark analysis. [Figure 1]
Discussion: There was no association between receipt of > 3 doses of infliximab and worsened cancer outcomes, including PFS and OS, in our series, failing to detect cancer risk with longer term infliximab therapy. However, given the limitations of our study, including small sample size, single-center analysis, retrospective design, and lack of diversity, further prospective studies are warranted to confirm these findings and optimize infliximab dosing strategies.

Figure: Figure 1:Landmark analysis by infliximab cycles (≤3 and >3):
(Figure 1A) Time to progression (TTP), with a median follow-up time of 703.5 days (IQR: 529.5 - 1190.5) among those without progression.
(Figure 1B) Progression-free survival (PFS), with a median follow-up time of 676 days (IQR: 481 - 1202) among those still alive without progression.
(Figure 1C) Overall survival (OS), with a median follow-up time of 709 days (IQR: 371 - 1204) among those still alive.
(Figure 1A) Time to progression (TTP), with a median follow-up time of 703.5 days (IQR: 529.5 - 1190.5) among those without progression.
(Figure 1B) Progression-free survival (PFS), with a median follow-up time of 676 days (IQR: 481 - 1202) among those still alive without progression.
(Figure 1C) Overall survival (OS), with a median follow-up time of 709 days (IQR: 371 - 1204) among those still alive.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Rupayan Kundu indicated no relevant financial relationships.
Arjun Chatterjee indicated no relevant financial relationships.
Emily Zabor indicated no relevant financial relationships.
James Isaacs indicated no relevant financial relationships.
Thach-Giao Truong: Erasca – Institutional trial funding. Merck – Advisory Committee/Board Member, Institutional trial support. Novartis – Medical Writing fees. Pfizer – Advisory Committee/Board Member, institutional grant support, teaching fees. Regeneron – Institutional trial support.
Joseph Sleiman indicated no relevant financial relationships.
Pauline Funchain indicated no relevant financial relationships.
Jessica Philpott: Abbvie – Speakers Bureau.
Lucy Kennedy indicated no relevant financial relationships.
Rupayan Kundu, MD1, Arjun Chatterjee, MD1, Emily Zabor, 1, James Isaacs, MD1, Thach-Giao Truong, MD1, Joseph Sleiman, MD2, Pauline Funchain, MD3, Jessica Philpott, MD, PhD1, Lucy Kennedy, MD1. P0913 - How Many Doses of Infliximab Should Be Used in Immune Checkpoint Inhibitor-Induced Colitis? – A Retrospective Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
