Sunday Poster Session
Category: IBD
P0922 - Treatment Utilization and Sequencing in Patients With Ulcerative Colitis and Crohn's Disease
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Ayush Patel, MS, PhD
Teva Branded Pharmaceuticals Products R&D, Inc.
West Chester, PA
Presenting Author(s)
Vipul Jairath, MBChB1, Handing Xie, MS2, Jing Wang, MPH3, Dalbir Dhiraj, BSc, MSc, PhD4, Ayush Patel, MS, PhD5
1Western University, London, ON, Canada; 2Teva Branded Pharmaceutical Products R&D, Inc., West Chester, PA; 3KMK Consulting Inc., Morristown, NJ; 4Teva UK Limited, Harlow, England, United Kingdom; 5Teva Branded Pharmaceuticals Products R&D, Inc., West Chester, PA
Introduction: Ulcerative colitis (UC) and Crohn’s disease (CD), collectively referred to as inflammatory bowel disease (IBD), are chronic disorders of the gastrointestinal tract typically marked by remission and relapse.1 Recently new therapies have become available to treat IBD though there is a paucity of data on their use in practice. This study assessed treatment sequencing in patients with UC & CD newly initiated on advanced therapies (AT) or immunosuppressants (IMS).
Methods: This retrospective US claims database (Merative™ Marketscan®) study assessed treatment sequencing over a 24-month period in adults with UC & CD newly initiated on an AT or IMS therapy between January 1, 2017-March 31, 2021. FDA-approved therapies through March 2021 were included. Index date was defined as the first prescription date of AT or IMS. Patients were required to be continuously enrolled for at least 12-months pre-index (baseline period) and 24-months post-index (follow-up period). Descriptive statistics were used to assess baseline characteristics & follow-up treatment sequences.
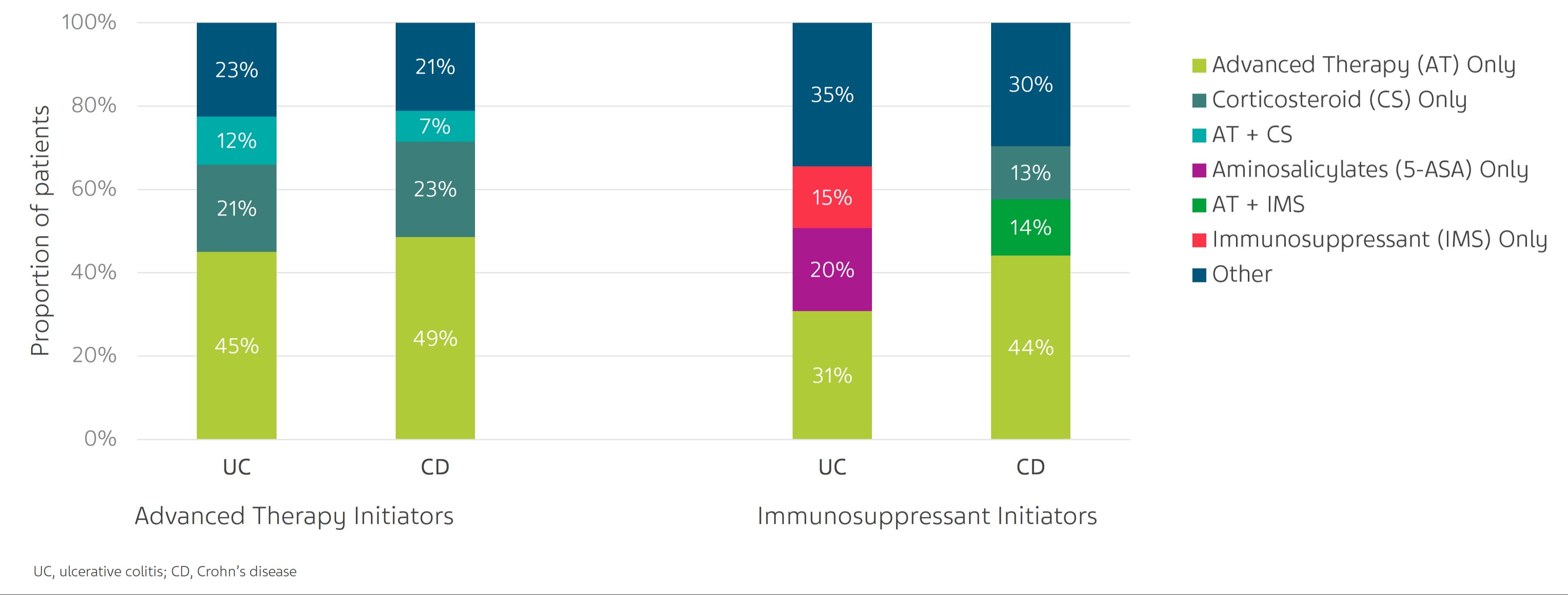
Results: Of the 10,585 IBD patients identified, 4651 had UC & 5934 had CD. Baseline characteristics are described in Table 1. Among AT initiators (UC: 3076; CD: 4043), 79% UC & 84% CD patients received AT-only; the remainder received AT + corticosteroids (CS) and/or IMS. Of the AT initiators, ~47% UC & ~40% CD patients received subsequent second-line therapy (Fig 1). Among IMS initiators (UC: 1575; CD: 1891), 58% UC & 62% CD patients were treated with IMS-only; the remainder on IMS+CS and/or AT. Almost 80% UC & 79% CD IMS initiators received subsequent second-line therapy (Fig 1).
Discussion: Whilst there is an armamentarium of options available to treat UC & CD, this real-world study shows that regardless of AT or IMS, many patients receive combination and/or multiple lines of therapy, as previously reported.2 This highlights that a substantial unmet need for a durable, effective, and tolerable treatment option persists.
References
1. Wang R, et al. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023;13:e065186.
2. Triantafillidis JK, et al. Combination treatment of inflammatory bowel disease: Present status and future perspectives. World J Gastroenterol. 2024 Apr 21;30(15):2068-2080.
Disclosures: The study was funded by Teva Pharmaceuticals; publication supported by Teva-Sanofi Alliance.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Vipul Jairath, MBChB1, Handing Xie, MS2, Jing Wang, MPH3, Dalbir Dhiraj, BSc, MSc, PhD4, Ayush Patel, MS, PhD5. P0922 - Treatment Utilization and Sequencing in Patients With Ulcerative Colitis and Crohn's Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Western University, London, ON, Canada; 2Teva Branded Pharmaceutical Products R&D, Inc., West Chester, PA; 3KMK Consulting Inc., Morristown, NJ; 4Teva UK Limited, Harlow, England, United Kingdom; 5Teva Branded Pharmaceuticals Products R&D, Inc., West Chester, PA
Introduction: Ulcerative colitis (UC) and Crohn’s disease (CD), collectively referred to as inflammatory bowel disease (IBD), are chronic disorders of the gastrointestinal tract typically marked by remission and relapse.1 Recently new therapies have become available to treat IBD though there is a paucity of data on their use in practice. This study assessed treatment sequencing in patients with UC & CD newly initiated on advanced therapies (AT) or immunosuppressants (IMS).
Methods: This retrospective US claims database (Merative™ Marketscan®) study assessed treatment sequencing over a 24-month period in adults with UC & CD newly initiated on an AT or IMS therapy between January 1, 2017-March 31, 2021. FDA-approved therapies through March 2021 were included. Index date was defined as the first prescription date of AT or IMS. Patients were required to be continuously enrolled for at least 12-months pre-index (baseline period) and 24-months post-index (follow-up period). Descriptive statistics were used to assess baseline characteristics & follow-up treatment sequences.
Results: Of the 10,585 IBD patients identified, 4651 had UC & 5934 had CD. Baseline characteristics are described in Table 1. Among AT initiators (UC: 3076; CD: 4043), 79% UC & 84% CD patients received AT-only; the remainder received AT + corticosteroids (CS) and/or IMS. Of the AT initiators, ~47% UC & ~40% CD patients received subsequent second-line therapy (Fig 1). Among IMS initiators (UC: 1575; CD: 1891), 58% UC & 62% CD patients were treated with IMS-only; the remainder on IMS+CS and/or AT. Almost 80% UC & 79% CD IMS initiators received subsequent second-line therapy (Fig 1).
Discussion: Whilst there is an armamentarium of options available to treat UC & CD, this real-world study shows that regardless of AT or IMS, many patients receive combination and/or multiple lines of therapy, as previously reported.2 This highlights that a substantial unmet need for a durable, effective, and tolerable treatment option persists.
References
1. Wang R, et al. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023;13:e065186.
2. Triantafillidis JK, et al. Combination treatment of inflammatory bowel disease: Present status and future perspectives. World J Gastroenterol. 2024 Apr 21;30(15):2068-2080.
Disclosures: The study was funded by Teva Pharmaceuticals; publication supported by Teva-Sanofi Alliance.

Figure: Figure 1. Second-line treatment regimens
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Employee, Grant/Research Support, Speakers Bureau. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Advisory Committee/Board Member, Consultant. Enthera – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Advisory Committee/Board Member, Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Advisory Committee/Board Member, Consultant. JAMP – Advisory Committee/Board Member, Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. London Health Sciences Centre – Employee. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Advisory Committee/Board Member, Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. SCOPE – Advisory Committee/Board Member, Consultant. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Synedgen – Advisory Committee/Board Member, Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TD Securities – Advisory Committee/Board Member, Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Handing Xie: Teva Branded Pharmaceuticals Products R&D, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Jing Wang: Teva Branded Pharmaceuticals Products R&D, Inc. – Consultant.
Dalbir Dhiraj: Teva UK Limited – Employee, Stock-publicly held company(excluding mutual/index funds).
Ayush Patel: Teva Branded Pharmaceuticals Products R&D, Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Vipul Jairath, MBChB1, Handing Xie, MS2, Jing Wang, MPH3, Dalbir Dhiraj, BSc, MSc, PhD4, Ayush Patel, MS, PhD5. P0922 - Treatment Utilization and Sequencing in Patients With Ulcerative Colitis and Crohn's Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
