Sunday Poster Session
Category: IBD
P0925 - A Multi-Center Study of the Outcomes of Risankizumab Use Stratified by Prior Ustekinumab Exposure in Patients With Crohn’s Disease: A Real-World Experience
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- RS
Raina Shivashankar, MD
Thomas Jefferson University Hospital
Philadelphia, PA
Presenting Author(s)
Scott Manski, MD1, Michael Andreone, MD2, Shreya Swaminathan, BS3, Fernando Cordero-Baez, MD4, Jonathan Colon Sanchez, MD4, Jenna Kantor, MS5, Scott Keith, PhD5, Eugenia Shmidt, MD2, Raina Shivashankar, MD4
1University of South Florida, Tampa, FL; 2University of Minnesota, Minneapolis, MN; 3Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA; 4Thomas Jefferson University Hospital, Philadelphia, PA; 5Thomas Jefferson University, Philadelphia, PA
Introduction: Inflammatory bowel disease (IBD) patients (pts) may have inadequate response to biologic therapy. Risankizumab (RZA) was FDA approved in 2022 for the treatment of moderate-severe Crohn’s disease (CD). In this multicenter study, we sought to describe the outcomes of RZA use in CD pts and to compare those who were ustekinumab (UST)-exposed versus UST-naive.
Methods: A retrospective review of electronic health records between 8/2022 and 1/2024 was conducted. Adult pts with a history of CD who started RZA between 8/2022 and 8/2023 were included. Pt demographics and IBD/biologic history were collected. Harvey Bradshaw Index (HBI) scores and objective markers (fecal calprotectin [FCP]) were assessed through 20 wks. Adverse events (AEs) during the study period were also documented. Paired t-test and McNemar’s test were used.
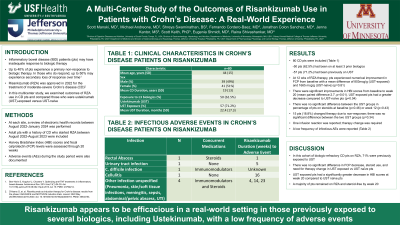
Results: We identified 80 CD pts treated with RZA (avg age 44yrs [SD: 15yrs], 51% female, mean CD duration 19 yrs [SD:13 yrs], 55% with perianal disease). 50 pts (62.5%) had been on at least 3 biologics prior to RZA start. 57 (71.2%) pts had been on UST (mean duration 22.4 mo [SD: 17.3 mo]). At 12 wks of RZA therapy, pts experienced numerical improvement in FCP from baseline with a mean difference of 803mcg/g (UST-exposed) and 1665 mcg/g (UST-naïve) (p=0.81). There were significant improvements in HBI scores from baseline to week 20 (mean paired difference 2.7, p=0.01), and UST-exposed pts had a greater decrease compared to UST-naïve pts (p=0.04). There was no significant difference in percentage of pts on steroids at baseline (p=0.46) or week 12 (p=0.43) between the two groups. 15 pts (18.8%) changed therapy due to non-response, with no significant difference between the two UST groups (p=0.34). One infusion reaction was reported in the total cohort, and therapy change was required. Please refer to Table 1 for details on infectious AEs in the total cohort.
Discussion: In this cohort of biologic-refractory CD pts on RZA, 71% were exposed to UST. There was no significant difference in FCP decrease, steroid use, and need for therapy change in pts previously exposed to UST versus not. UST exposed pts had a significantly greater decrease in HBI scores at week 20 compared to UST naïve pts. A majority of pts remained on RZA and were steroid-free by week 20. RZA appears to be efficacious in a real-world setting in those previously exposed to UST; our data suggest a low frequency of AEs. We aim to expand upon these findings in a larger cohort of pts.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Scott Manski, MD1, Michael Andreone, MD2, Shreya Swaminathan, BS3, Fernando Cordero-Baez, MD4, Jonathan Colon Sanchez, MD4, Jenna Kantor, MS5, Scott Keith, PhD5, Eugenia Shmidt, MD2, Raina Shivashankar, MD4. P0925 - A Multi-Center Study of the Outcomes of Risankizumab Use Stratified by Prior Ustekinumab Exposure in Patients With Crohn’s Disease: A Real-World Experience, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of South Florida, Tampa, FL; 2University of Minnesota, Minneapolis, MN; 3Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA; 4Thomas Jefferson University Hospital, Philadelphia, PA; 5Thomas Jefferson University, Philadelphia, PA
Introduction: Inflammatory bowel disease (IBD) patients (pts) may have inadequate response to biologic therapy. Risankizumab (RZA) was FDA approved in 2022 for the treatment of moderate-severe Crohn’s disease (CD). In this multicenter study, we sought to describe the outcomes of RZA use in CD pts and to compare those who were ustekinumab (UST)-exposed versus UST-naive.
Methods: A retrospective review of electronic health records between 8/2022 and 1/2024 was conducted. Adult pts with a history of CD who started RZA between 8/2022 and 8/2023 were included. Pt demographics and IBD/biologic history were collected. Harvey Bradshaw Index (HBI) scores and objective markers (fecal calprotectin [FCP]) were assessed through 20 wks. Adverse events (AEs) during the study period were also documented. Paired t-test and McNemar’s test were used.
Results: We identified 80 CD pts treated with RZA (avg age 44yrs [SD: 15yrs], 51% female, mean CD duration 19 yrs [SD:13 yrs], 55% with perianal disease). 50 pts (62.5%) had been on at least 3 biologics prior to RZA start. 57 (71.2%) pts had been on UST (mean duration 22.4 mo [SD: 17.3 mo]). At 12 wks of RZA therapy, pts experienced numerical improvement in FCP from baseline with a mean difference of 803mcg/g (UST-exposed) and 1665 mcg/g (UST-naïve) (p=0.81). There were significant improvements in HBI scores from baseline to week 20 (mean paired difference 2.7, p=0.01), and UST-exposed pts had a greater decrease compared to UST-naïve pts (p=0.04). There was no significant difference in percentage of pts on steroids at baseline (p=0.46) or week 12 (p=0.43) between the two groups. 15 pts (18.8%) changed therapy due to non-response, with no significant difference between the two UST groups (p=0.34). One infusion reaction was reported in the total cohort, and therapy change was required. Please refer to Table 1 for details on infectious AEs in the total cohort.
Discussion: In this cohort of biologic-refractory CD pts on RZA, 71% were exposed to UST. There was no significant difference in FCP decrease, steroid use, and need for therapy change in pts previously exposed to UST versus not. UST exposed pts had a significantly greater decrease in HBI scores at week 20 compared to UST naïve pts. A majority of pts remained on RZA and were steroid-free by week 20. RZA appears to be efficacious in a real-world setting in those previously exposed to UST; our data suggest a low frequency of AEs. We aim to expand upon these findings in a larger cohort of pts.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Scott Manski indicated no relevant financial relationships.
Michael Andreone indicated no relevant financial relationships.
Shreya Swaminathan indicated no relevant financial relationships.
Fernando Cordero-Baez indicated no relevant financial relationships.
Jonathan Colon Sanchez indicated no relevant financial relationships.
Jenna Kantor indicated no relevant financial relationships.
Scott Keith indicated no relevant financial relationships.
Eugenia Shmidt: BMS – Grant/Research Support. UCB – Grant/Research Support.
Raina Shivashankar: Abbvie – Speakers Bureau. BMS – Speakers Bureau. Janssen – Grant/Research Support.
Scott Manski, MD1, Michael Andreone, MD2, Shreya Swaminathan, BS3, Fernando Cordero-Baez, MD4, Jonathan Colon Sanchez, MD4, Jenna Kantor, MS5, Scott Keith, PhD5, Eugenia Shmidt, MD2, Raina Shivashankar, MD4. P0925 - A Multi-Center Study of the Outcomes of Risankizumab Use Stratified by Prior Ustekinumab Exposure in Patients With Crohn’s Disease: A Real-World Experience, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
