Sunday Poster Session
Category: IBD
P0831 - Real-World Outcomes of a Mandatory Adalimumab Non-Medical Biosimilar Switch for Patients With Inflammatory Bowel Disease: A Single Center Retrospective Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- TH
Thomas Hoang, MD
University of British Columbia
Burnaby, BC, Canada
Presenting Author(s)
Thomas Hoang, MD1, Jeremy Liu Chen Kiow, MD2, Harjot Bedi, MD3, Zhina Majdzadeh Ardekani, BSc3, Daniel Rosenfeld, 3, Marica Reise-Filteau, MD3, Brian Bressler, MS, MD3, Yvette Leung, MD3, Greg Rosenfeld, MD3
1University of British Columbia, Burnaby, BC, Canada; 2University of British Columbia, IBD Centre of BC, Vanouver, BC, Canada; 3University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada
Introduction: Adalimumab (ADA) is a tumor necrosis factor alpha (TNF-α) antagonist that is approved for the treatment of inflammatory bowel disease (IBD) in Canada, first approved under the trade name Humira. Between April to September 2021, the province of British Columbia implemented a mandatory non-medical switch of Humira to one of five approved biosimilars. While existing evidence supports the safety and efficacy of biosimilars, data regarding non-medical switching of ADA in IBD patients remain sparse.
Methods: A retrospective observational study was conducted using a patient database from IBD Centre of BC, a tertiary referral centre associated with St. Paul’s Hospital in British Columbia, Canada. The disease outcomes of patients on Humira who switched to one of the five ADA biosimilars were compared to patients who remained on Humira through compassionate support or private pay. The primary outcome was treatment persistence at 30 months post-switch assessed by Kaplan-Meier survival analysis. Secondary outcomes included frequency of reasons for ADA discontinuation, loss of response rates, adverse events, as well as clinical and biochemical remission status. Patients who were initiated directly on a biosimilar were excluded.
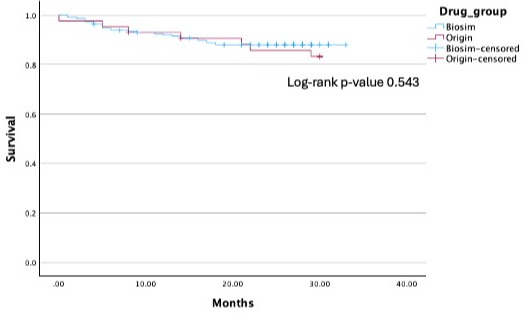
Results: Patients in the originator (n=43) and biosimilar (n=228) groups displayed similar demographics and baseline disease characteristics. By the study endpoint of 30 months, there was no difference in the rate of treatment persistence in either group (n=36, 83.7% originators vs. n= 201, 88.2% biosimilars, p=0.45). Treatment persistence assessed by Kaplan survival analysis demonstrated similar rates of discontinuation between both study groups (log-rank p-value = 0.537). There was a numerical but not statistically significant difference in rates of adverse events between either group (39.5 originator vs. 28.9% biosimilars, p=0.206). This included comparable rates of loss of response (27.9 vs. 17.5%) or adverse events (11.6 vs. 11.4%) between the originator and biosimilar cohorts. C-reactive protein and fecal calprotectin levels were also similar one year pre- and post-switch.
Discussion: Non-medical switching of the ADA originator to one of five biosimilars did not result in differences in treatment persistence or adverse outcomes compared to originator molecule continuation for patients with IBD. These data will help inform patients and physicians in jurisdictions currently undergoing biosimilar switching.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Thomas Hoang, MD1, Jeremy Liu Chen Kiow, MD2, Harjot Bedi, MD3, Zhina Majdzadeh Ardekani, BSc3, Daniel Rosenfeld, 3, Marica Reise-Filteau, MD3, Brian Bressler, MS, MD3, Yvette Leung, MD3, Greg Rosenfeld, MD3. P0831 - Real-World Outcomes of a Mandatory Adalimumab Non-Medical Biosimilar Switch for Patients With Inflammatory Bowel Disease: A Single Center Retrospective Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of British Columbia, Burnaby, BC, Canada; 2University of British Columbia, IBD Centre of BC, Vanouver, BC, Canada; 3University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada
Introduction: Adalimumab (ADA) is a tumor necrosis factor alpha (TNF-α) antagonist that is approved for the treatment of inflammatory bowel disease (IBD) in Canada, first approved under the trade name Humira. Between April to September 2021, the province of British Columbia implemented a mandatory non-medical switch of Humira to one of five approved biosimilars. While existing evidence supports the safety and efficacy of biosimilars, data regarding non-medical switching of ADA in IBD patients remain sparse.
Methods: A retrospective observational study was conducted using a patient database from IBD Centre of BC, a tertiary referral centre associated with St. Paul’s Hospital in British Columbia, Canada. The disease outcomes of patients on Humira who switched to one of the five ADA biosimilars were compared to patients who remained on Humira through compassionate support or private pay. The primary outcome was treatment persistence at 30 months post-switch assessed by Kaplan-Meier survival analysis. Secondary outcomes included frequency of reasons for ADA discontinuation, loss of response rates, adverse events, as well as clinical and biochemical remission status. Patients who were initiated directly on a biosimilar were excluded.
Results: Patients in the originator (n=43) and biosimilar (n=228) groups displayed similar demographics and baseline disease characteristics. By the study endpoint of 30 months, there was no difference in the rate of treatment persistence in either group (n=36, 83.7% originators vs. n= 201, 88.2% biosimilars, p=0.45). Treatment persistence assessed by Kaplan survival analysis demonstrated similar rates of discontinuation between both study groups (log-rank p-value = 0.537). There was a numerical but not statistically significant difference in rates of adverse events between either group (39.5 originator vs. 28.9% biosimilars, p=0.206). This included comparable rates of loss of response (27.9 vs. 17.5%) or adverse events (11.6 vs. 11.4%) between the originator and biosimilar cohorts. C-reactive protein and fecal calprotectin levels were also similar one year pre- and post-switch.
Discussion: Non-medical switching of the ADA originator to one of five biosimilars did not result in differences in treatment persistence or adverse outcomes compared to originator molecule continuation for patients with IBD. These data will help inform patients and physicians in jurisdictions currently undergoing biosimilar switching.

Figure: Fig 1 Kaplan-Meier survival analysis does not demonstrate any statistically significant difference in treatment persistence by 30-months of patients who remain on the originator vs. those who underwent the biosimilar switch (log-rank p = 0.543). Seven discontinuations (16.3%) occurred in the originator group, compared to 27 (11.8%) in the biosimilars group. Specific reasons for treatment discontinuations are summarized in Table 2.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Thomas Hoang indicated no relevant financial relationships.
Jeremy Liu Chen Kiow indicated no relevant financial relationships.
Harjot Bedi indicated no relevant financial relationships.
Zhina Majdzadeh Ardekani indicated no relevant financial relationships.
Daniel Rosenfeld indicated no relevant financial relationships.
Marica Reise-Filteau indicated no relevant financial relationships.
Brian Bressler: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Alimentiv – Advisory Committee/Board Member. Allergan – Advisory Committee/Board Member. Amgen – Advisory Committee/Board Member, Grant/Research Support. AMT – Advisory Committee/Board Member. Bausch Health – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Grant/Research Support. Celgene – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Eupraxia – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. Genentech/Roche – Advisory Committee/Board Member, Grant/Research Support. Gilead – Advisory Committee/Board Member. GSK – Grant/Research Support. Iterative Scopes – Advisory Committee/Board Member. Jamp – Advisor or Review Panel Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Merck – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisor or Review Panel Member. Mylan – Advisor or Review Panel Member. Novartis – Advisory Committee/Board Member, Speakers Bureau. Organon – Advisory Committee/Board Member, Speakers Bureau. Pendopharm – Advisor or Review Panel Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Protagonist – Advisor or Review Panel Member. Qu Biologic – Grant/Research Support, Stock Options. Sandoz – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau. Viatris – Advisor or Review Panel Member.
Yvette Leung: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Amgen – Advisory Committee/Board Member, Speakers Bureau. BMS – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Organon – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Greg Rosenfeld: Abbvie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Amgen – Advisory Committee/Board Member, Speakers Bureau. Crohn’s and Colitis Canada – Grant/Research Support. Ferring – Advisory Committee/Board Member, Grant/Research Support, Royalties. Frensius-Kabi – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Grant/Research Support. Merck – Advisory Committee/Board Member, Speakers Bureau. Organon – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau. Viatris – Advisory Committee/Board Member, Speakers Bureau.
Thomas Hoang, MD1, Jeremy Liu Chen Kiow, MD2, Harjot Bedi, MD3, Zhina Majdzadeh Ardekani, BSc3, Daniel Rosenfeld, 3, Marica Reise-Filteau, MD3, Brian Bressler, MS, MD3, Yvette Leung, MD3, Greg Rosenfeld, MD3. P0831 - Real-World Outcomes of a Mandatory Adalimumab Non-Medical Biosimilar Switch for Patients With Inflammatory Bowel Disease: A Single Center Retrospective Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
