Sunday Poster Session
Category: General Endoscopy
P0662 - Perioperative GLP-1 Receptor Agonist Impact on Endoscopy and Colonoscopy Outcomes: A Systematic Review and Meta-Analysis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Yahya Alsawaf, MD
MedStar Health
Towson, MD
Presenting Author(s)
Yahya Alsawaf, MD1, Samer Saadi, MD2, Zein Tarakji, MD2, Moustafa Hegazi, MD2, Muhamad Oum, MD3, Siddharth Singh, MD4, M. Hassan Murad, MD2
1MedStar Health, Towson, MD; 2Mayo Clinic, Rochester, MN; 3Saint Agnes Medical Center, Fresno, CA; 4University of California San Diego, La Jolla, CA
Introduction: Glucagon-like peptide-1 receptor agonists (GLP1-RAs) are effective in managing type 2 diabetes mellitus and promoting weight loss, but their perioperative safety remains a concern. This systematic review evaluates the effects of GLP1-RAs on perioperative complications of endoscopy (EGD) and colonoscopy procedures.
Methods: We comprehensively searched several databases, from each database's inception to April 2024, in the English language. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. Controlled vocabulary supplemented with keywords was used to search for perioperative complications in patients taking GLP-1 agonists. This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statements. We included RCTs and observational studies.
Results: Our electronic search identified 2291 citations, from which seven studies reported perioperative complications in EGD and colonoscopy were included.
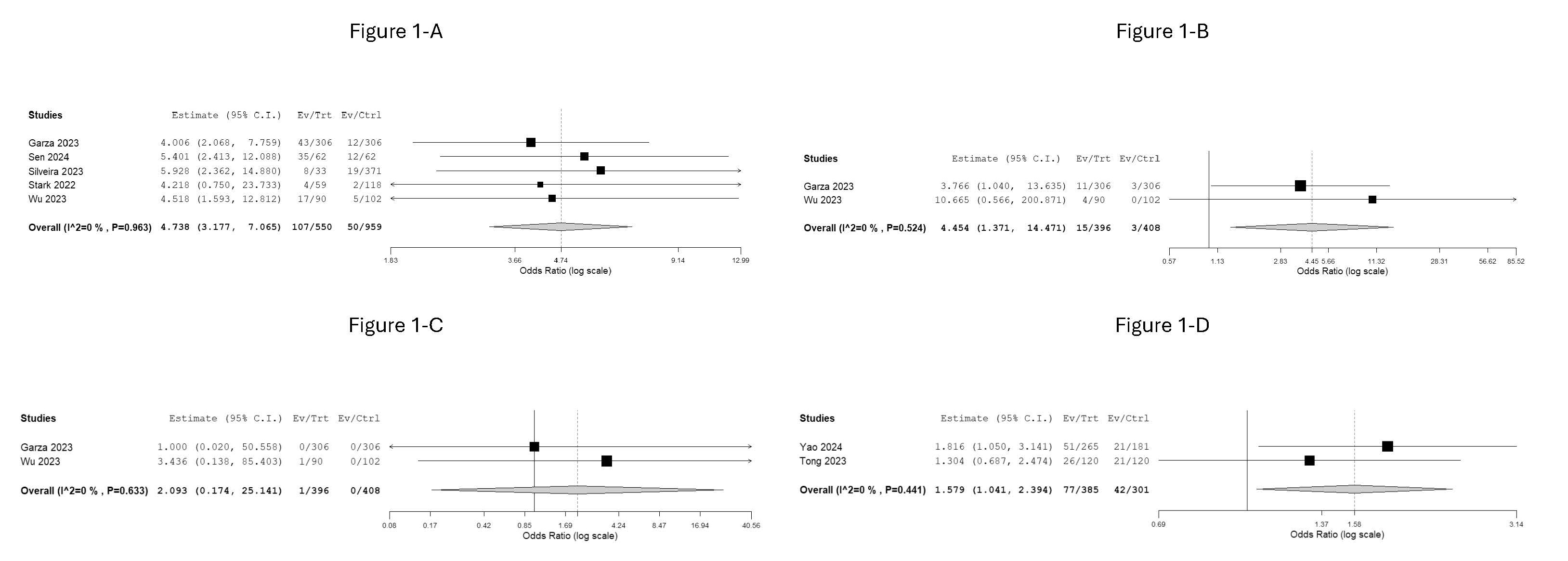
EGD: Five observational studies revealed an increased risk of retained gastric content (RGC) in EGD with GLP-1 RAs use (OR: 4.738; 95% CI, 3.177-7.065; p< 0.001) (Figure 1-A). While two studies reported a higher risk of intubation during EGD with GLP1-RA use (OR: 4.454; 95% CI, 1.371-14.471; p=0.013) (Figure 1-B), they found no significant increase in pulmonary aspiration risk (OR: 2.093; 95% CI, 0.174-25.141; p=0.560) (Figure 1-C). One study showed no increased risk of needing to repeat EGD with GLP1-RAs use (OR: 1.992; 95% CI, 0.039-101.617).
Colonoscopy: Two studies indicated an increased risk of inadequate bowel cleaning with GLP-1 RAs use (OR: 1.579; 95% CI, 1.104-2.394; p=0.032) (Figure 1-D). Notably, only one study reported an increase in the need for repeat colonoscopy (OR: 1.816; 95% CI, 1.05-3.141). One study found no association between GLP-1 RAs (liraglutide) use and hypoglycemia during colonoscopy (OR: 0.881; 95% CI, 0.328-2.366).
Discussion: GLP1-RAs use was associated with an increased risk of RGC and inadequate bowel preparation, which impacted the quality of EGD and colonoscopy. Although some clinicians recommend withholding GLP1-RAs preoperatively, evidence is insufficient. Further robust research is needed to assess perioperative risks and determine whether modifying medication or fasting protocols can mitigate these adverse events.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Yahya Alsawaf, MD1, Samer Saadi, MD2, Zein Tarakji, MD2, Moustafa Hegazi, MD2, Muhamad Oum, MD3, Siddharth Singh, MD4, M. Hassan Murad, MD2. P0662 - Perioperative GLP-1 Receptor Agonist Impact on Endoscopy and Colonoscopy Outcomes: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1MedStar Health, Towson, MD; 2Mayo Clinic, Rochester, MN; 3Saint Agnes Medical Center, Fresno, CA; 4University of California San Diego, La Jolla, CA
Introduction: Glucagon-like peptide-1 receptor agonists (GLP1-RAs) are effective in managing type 2 diabetes mellitus and promoting weight loss, but their perioperative safety remains a concern. This systematic review evaluates the effects of GLP1-RAs on perioperative complications of endoscopy (EGD) and colonoscopy procedures.
Methods: We comprehensively searched several databases, from each database's inception to April 2024, in the English language. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. Controlled vocabulary supplemented with keywords was used to search for perioperative complications in patients taking GLP-1 agonists. This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statements. We included RCTs and observational studies.
Results: Our electronic search identified 2291 citations, from which seven studies reported perioperative complications in EGD and colonoscopy were included.
EGD: Five observational studies revealed an increased risk of retained gastric content (RGC) in EGD with GLP-1 RAs use (OR: 4.738; 95% CI, 3.177-7.065; p< 0.001) (Figure 1-A). While two studies reported a higher risk of intubation during EGD with GLP1-RA use (OR: 4.454; 95% CI, 1.371-14.471; p=0.013) (Figure 1-B), they found no significant increase in pulmonary aspiration risk (OR: 2.093; 95% CI, 0.174-25.141; p=0.560) (Figure 1-C). One study showed no increased risk of needing to repeat EGD with GLP1-RAs use (OR: 1.992; 95% CI, 0.039-101.617).
Colonoscopy: Two studies indicated an increased risk of inadequate bowel cleaning with GLP-1 RAs use (OR: 1.579; 95% CI, 1.104-2.394; p=0.032) (Figure 1-D). Notably, only one study reported an increase in the need for repeat colonoscopy (OR: 1.816; 95% CI, 1.05-3.141). One study found no association between GLP-1 RAs (liraglutide) use and hypoglycemia during colonoscopy (OR: 0.881; 95% CI, 0.328-2.366).
Discussion: GLP1-RAs use was associated with an increased risk of RGC and inadequate bowel preparation, which impacted the quality of EGD and colonoscopy. Although some clinicians recommend withholding GLP1-RAs preoperatively, evidence is insufficient. Further robust research is needed to assess perioperative risks and determine whether modifying medication or fasting protocols can mitigate these adverse events.

Figure: Figure 1. Forest plot of the odds ratio (OR) for perioperative complications associated with GLP-1 RA use compared to non-use in patients undergoing EGD (A, B, C) or colonoscopy (D). The analysis used a random-effects model. The diamond represents the pooled OR and its 95% confidence interval. Heterogeneity was assessed using the I^2 statistic.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Yahya Alsawaf indicated no relevant financial relationships.
Samer Saadi indicated no relevant financial relationships.
Zein Tarakji indicated no relevant financial relationships.
Moustafa Hegazi indicated no relevant financial relationships.
Muhamad Oum indicated no relevant financial relationships.
Siddharth Singh: Pfizer – Advisor or Review Panel Member, Grant/Research Support.
M. Hassan Murad indicated no relevant financial relationships.
Yahya Alsawaf, MD1, Samer Saadi, MD2, Zein Tarakji, MD2, Moustafa Hegazi, MD2, Muhamad Oum, MD3, Siddharth Singh, MD4, M. Hassan Murad, MD2. P0662 - Perioperative GLP-1 Receptor Agonist Impact on Endoscopy and Colonoscopy Outcomes: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
