Sunday Poster Session
Category: Functional Bowel Disease
P0615 - Efficacy, Safety and Time to Response of Linaclotide in Adult Patients With Chronic Idiopathic Constipation: A Post Hoc Subgroup Analysis by Age
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Lin Chang, MD
Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA
Los Angeles, CA
Presenting Author(s)
Lin Chang, MD1, Darren M Brenner, MD2, Wendy Chen, PharmD3, Niha Yerneni, PharmD, MPH4, James Wu, PhD4, Paul Feuerstadt, MD5, Kyle Staller, MD, MPH6
1Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA, Los Angeles, CA; 2Northwestern University Feinberg School of Medicine, North Chicago, IL; 3AbbVie, Inc., North Chicago, IL; 4Ironwood Pharmaceuticals, Inc., Boston, MA; 5Yale School of Medicine, PACT Gastroenterology Center, Hamden, CT; 6Center for Neurointestinal Health, Massachusetts General Hospital, Boston, MA
Introduction: Chronic idiopathic constipation (CIC) is a functional gastrointestinal disorder that affects 5–19% of the US population, with prevalence increasing with age. This post hoc analysis of Phase 3 CIC study data assessed efficacy, safety and time to response of linaclotide (LIN) in adult patients stratified by age.
Methods: Data from four Phase 3 CIC randomized controlled trials (NCT00730015, NCT00765882, NCT01642914, NCT02291679) of patients treated with placebo (PBO), LIN 72 µg or LIN 145 µg were pooled and the study population was stratified by age (< 65 and ≥ 65 yrs). Inclusion criteria were similar across studies; patients met modified Rome II/III criteria for functional constipation. Baseline clinical characteristics were compared. Statistical analyses assessed the following for each group (PBO, LIN 72 µg and LIN 145 µg) by age: change from baseline in complete spontaneous bowel movement (CSBM) frequency, SBM frequency, stool consistency, severity of straining, abdominal symptoms (pain, discomfort and bloating) and constipation severity, and time to response for CSBM +≥ 1 frequency. Safety was evaluated across age groups. Primary efficacy endpoint results were previously published.
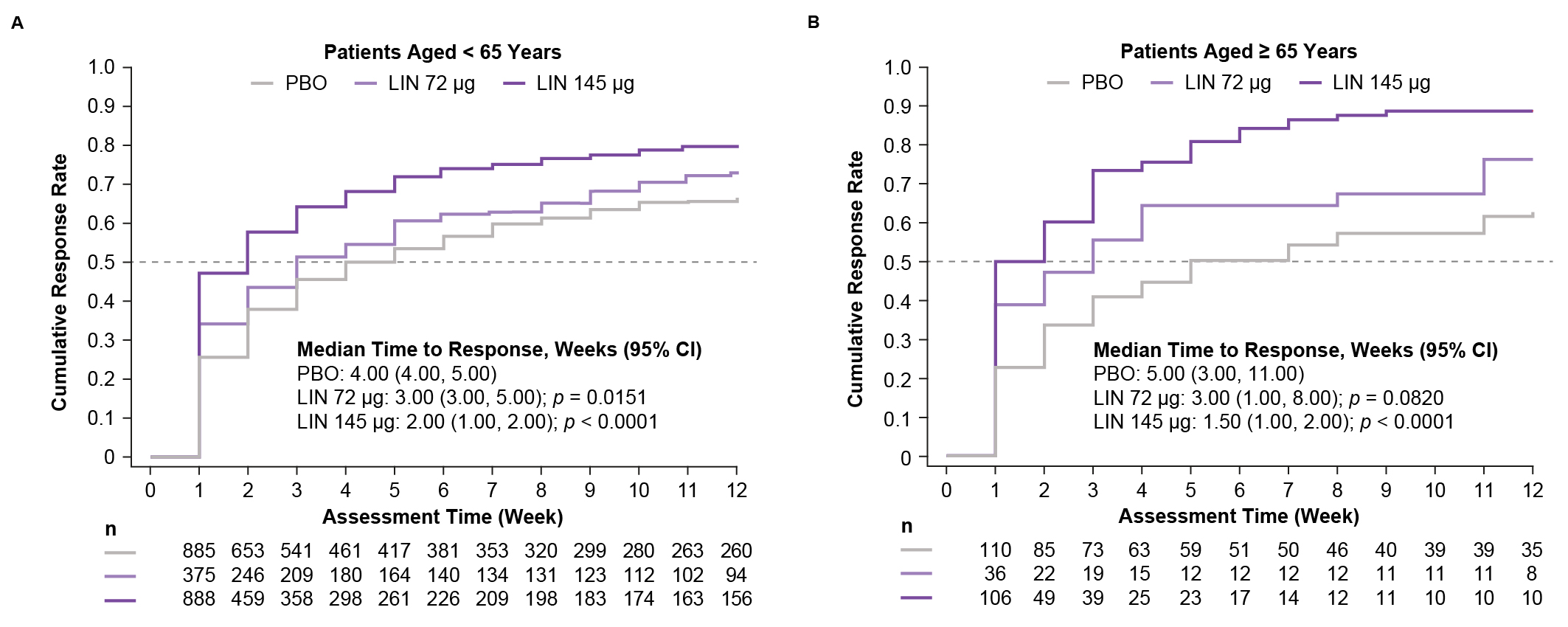
Results: Of the 2400 patients included, 2148 patients were aged < 65 yrs (mean age, female: PBO, 43.8 yrs, 86.6%; LIN 72 µg, 43.4 yrs, 77.3%; LIN 145 µg, 44.7 yrs, 86.1%) and 252 patients were aged ≥ 65 yrs (mean age, female: PBO, 70.3 yrs, 76.4%; LIN 72 µg, 71.4 yrs, 61.1%; LIN 145 µg, 70.3 yrs, 68.9%). Baseline bowel and abdominal symptoms were generally similar in both age groups. Over the 12-week treatment periods, LIN 72 µg and LIN 145 µg showed consistent trends in improvement from baseline in bowel and abdominal symptoms vs PBO in both age groups (Table). Median time to improvement from baseline in weekly CSBM +≥ 1 frequency was reduced with LIN 72 µg and LIN 145 µg vs PBO in patients aged < 65 and ≥ 65 yrs; Kaplan–Meier plots show separation between LIN and PBO in both age groups (Figure). Diarrhea was the most common treatment-emergent adverse event with LIN (< 65 yrs: PBO, 5.1%; LIN 72 µg, 19.2%; LIN 145 µg, 16.4%; ≥ 65 yrs: PBO, 6.4%; LIN 72 µg, 19.4%; LIN 145 µg, 21.7%).
Discussion: LIN-treated patients with CIC tended to show greater and faster improvement in bowel and abdominal symptoms compared with PBO and had similar safety profiles across age groups for both LIN doses. However, the small sample size for patients aged ≥ 65 yrs should be considered when interpreting these data.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Lin Chang, MD1, Darren M Brenner, MD2, Wendy Chen, PharmD3, Niha Yerneni, PharmD, MPH4, James Wu, PhD4, Paul Feuerstadt, MD5, Kyle Staller, MD, MPH6. P0615 - Efficacy, Safety and Time to Response of Linaclotide in Adult Patients With Chronic Idiopathic Constipation: A Post Hoc Subgroup Analysis by Age, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA, Los Angeles, CA; 2Northwestern University Feinberg School of Medicine, North Chicago, IL; 3AbbVie, Inc., North Chicago, IL; 4Ironwood Pharmaceuticals, Inc., Boston, MA; 5Yale School of Medicine, PACT Gastroenterology Center, Hamden, CT; 6Center for Neurointestinal Health, Massachusetts General Hospital, Boston, MA
Introduction: Chronic idiopathic constipation (CIC) is a functional gastrointestinal disorder that affects 5–19% of the US population, with prevalence increasing with age. This post hoc analysis of Phase 3 CIC study data assessed efficacy, safety and time to response of linaclotide (LIN) in adult patients stratified by age.
Methods: Data from four Phase 3 CIC randomized controlled trials (NCT00730015, NCT00765882, NCT01642914, NCT02291679) of patients treated with placebo (PBO), LIN 72 µg or LIN 145 µg were pooled and the study population was stratified by age (< 65 and ≥ 65 yrs). Inclusion criteria were similar across studies; patients met modified Rome II/III criteria for functional constipation. Baseline clinical characteristics were compared. Statistical analyses assessed the following for each group (PBO, LIN 72 µg and LIN 145 µg) by age: change from baseline in complete spontaneous bowel movement (CSBM) frequency, SBM frequency, stool consistency, severity of straining, abdominal symptoms (pain, discomfort and bloating) and constipation severity, and time to response for CSBM +≥ 1 frequency. Safety was evaluated across age groups. Primary efficacy endpoint results were previously published.
Results: Of the 2400 patients included, 2148 patients were aged < 65 yrs (mean age, female: PBO, 43.8 yrs, 86.6%; LIN 72 µg, 43.4 yrs, 77.3%; LIN 145 µg, 44.7 yrs, 86.1%) and 252 patients were aged ≥ 65 yrs (mean age, female: PBO, 70.3 yrs, 76.4%; LIN 72 µg, 71.4 yrs, 61.1%; LIN 145 µg, 70.3 yrs, 68.9%). Baseline bowel and abdominal symptoms were generally similar in both age groups. Over the 12-week treatment periods, LIN 72 µg and LIN 145 µg showed consistent trends in improvement from baseline in bowel and abdominal symptoms vs PBO in both age groups (Table). Median time to improvement from baseline in weekly CSBM +≥ 1 frequency was reduced with LIN 72 µg and LIN 145 µg vs PBO in patients aged < 65 and ≥ 65 yrs; Kaplan–Meier plots show separation between LIN and PBO in both age groups (Figure). Diarrhea was the most common treatment-emergent adverse event with LIN (< 65 yrs: PBO, 5.1%; LIN 72 µg, 19.2%; LIN 145 µg, 16.4%; ≥ 65 yrs: PBO, 6.4%; LIN 72 µg, 19.4%; LIN 145 µg, 21.7%).
Discussion: LIN-treated patients with CIC tended to show greater and faster improvement in bowel and abdominal symptoms compared with PBO and had similar safety profiles across age groups for both LIN doses. However, the small sample size for patients aged ≥ 65 yrs should be considered when interpreting these data.

Figure: Kaplan–Meier Analysis of Time to Improvement from Baseline in CSBM Frequency Rate of ≥ 1 by Age.
n is the number of patients at risk in the intention-to-treat population. An event was defined as the first week a patient had an increase from baseline in CSBM weekly rate of ≥ 1.
CI, confidence interval; CSBM, complete spontaneous bowel movement; LIN, linaclotide; PBO, placebo.
n is the number of patients at risk in the intention-to-treat population. An event was defined as the first week a patient had an increase from baseline in CSBM weekly rate of ≥ 1.
CI, confidence interval; CSBM, complete spontaneous bowel movement; LIN, linaclotide; PBO, placebo.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Lin Chang: AbbVie – Speaker. AnX Robotica – Grant/Research Support. Ardelyx – Advisory Committee/Board Member. Arena Pharmaceuticals – Grant/Research Support. Atmo Biosciences – Advisory Committee/Board Member. Bausch Health – Consultant, Speaker. FoodMarble Digestive Health – Consultant, Stock Options. Ironwood Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support. ModifyHealth – Stock Options. Trellus Health – Consultant, Stock-publicly held company(excluding mutual/index funds). Vibrant Advisory Board – Advisory Committee/Board Member. Vibrant Gastro – Advisory Committee/Board Member.
Darren M Brenner: AbbVie – Consultant, Speaker. Anji Pharmaceuticals – Consultant. Ardelyx – Advisor or Review Panel Member, Consultant, Speaker. Bayer – Consultant. Blueprint Medicines – Advisor or Review Panel Member. CinPhloro – Advisor or Review Panel Member, Consultant. Dr. Reddy's Laboratories – Consultant. Entrinsic Bioscience – Consultant. Gemelli Biotech – Advisor or Review Panel Member, Consultant. International Foundation for GI Disorders (IFFGD) – Board of Directors. Ironwood Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Laborie – Advisor or Review Panel Member. Mahana Therapeutics – Advisor or Review Panel Member, Consultant. Owlstone Medical – Advisor or Review Panel Member, Consultant, Stock-privately held company. Salix Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Vibrant Gastro – Advisor or Review Panel Member, Consultant.
Wendy Chen: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Niha Yerneni: Ironwood Pharmaceuticals – Employee, Stock-publicly held company(excluding mutual/index funds).
James Wu: Ironwood Pharmaceuticals – Employee, Stock-publicly held company(excluding mutual/index funds).
Paul Feuerstadt: Ferring Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Probiotech – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member, Speakers Bureau. Seres Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Takeda Pharmaceuticals – Advisory Committee/Board Member.
Kyle Staller: Anji Pharmaceuticals – Consultant. Ardelyx – Consultant, Grant/Research Support. GI Supply – Consultant. Mahana Therapeutics – Consultant. Restalsis Health – Consultant, Grant/Research Support. Salix – Consultant.
Lin Chang, MD1, Darren M Brenner, MD2, Wendy Chen, PharmD3, Niha Yerneni, PharmD, MPH4, James Wu, PhD4, Paul Feuerstadt, MD5, Kyle Staller, MD, MPH6. P0615 - Efficacy, Safety and Time to Response of Linaclotide in Adult Patients With Chronic Idiopathic Constipation: A Post Hoc Subgroup Analysis by Age, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
