Monday Poster Session
Category: Biliary/Pancreas
P1754 - Effect of Obeticholic Acid on Reduction and Normalization of Alanine Aminotransferase and Aspartate Aminotransferase in the Phase 3 POISE Trial in Primary Biliary Cholangitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- DW
Darren Wheeler, PhD
Intercept Pharmaceuticals, Inc.
Morristown, NJ
Presenting Author(s)
Robert G. Gish, MD1, Darren Wheeler, PhD2, Jing Li, PhD2, Christopher Gasink, MD2, David W. Victor, MD3
1Robert G. Gish Consultants, LLC, San Diego, CA; 2Intercept Pharmaceuticals, Inc., Morristown, NJ; 3Houston Methodist Hospital, Houston, TX
Introduction: Obeticholic acid (OCA) received accelerated approval for primary biliary cholangitis (PBC) based on improvements in alkaline phosphatase (ALP) and total bilirubin levels in the phase 3 POISE study. Elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are linked to an increased risk of negative outcomes in patients with PBC. We evaluated the effect of OCA on ALT and AST, including the proportion of patients normalizing these levels (ALT ≤ 41 U/L and AST ≤ 34 U/L) from abnormal baseline (BL) values.
Methods: In POISE, PBC patients with inadequate response or intolerance to ursodeoxycholic acid were randomized 1:1:1 to receive placebo (PBO), OCA titration (OCA 5 mg with option to titrate to 10 mg at month 6), or OCA 10 mg for 12 months. The Cochran–Mantel–Haenszel method was used to assess the association between OCA treatment and ALT or AST normalization among patients with elevated BL levels.
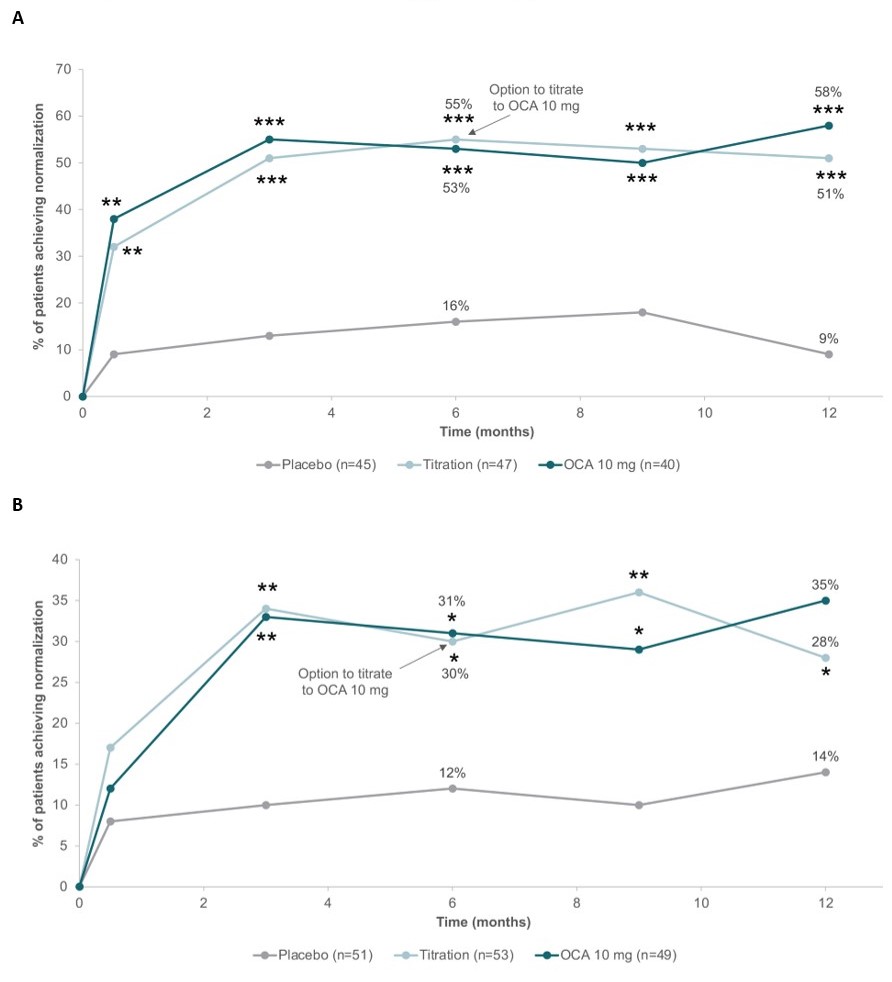
Results: In the intention-to-treat (ITT) population, mean BL ALT values were 56.0 U/L for PBO, 61.6 U/L for OCA titration, and 56.3 U/L for OCA 10 mg. Baseline AST levels were 48.8, 52.3, and 50.5 U/L for PBO, OCA titration, and OCA 10 mg, respectively. Among patients with ALT > 41 U/L at BL (61%), 16% (7/45) on PBO normalized at month 6 vs 55% (26/47) on OCA 5 mg and 53% (21/40) on OCA 10 mg (p ≤ 0.001 for both; Fig. 1A). By 2 weeks, 3 to 4 times more patients in both OCA groups normalized ALT (both p< 0.01 vs PBO). Among patients with AST > 34 U/L at BL (71%), normalization rates at month 6 were 12% (6/51) on PBO, 30% (16/53) on OCA 5 mg, and 31% (15/49) on OCA 10 mg (p < 0.05 for both; Fig. 1B). The proportions of patients normalizing ALT and AST on OCA were similar at month 12. In the ITT population, ALT and AST reductions were significant for both OCA groups vs PBO at all visits (all p < 0.001) as early as week 2. Least-squares mean changes from BL for ALT were −6.6 U/L (PBO), −18.9 U/L (OCA 5 mg), and −22.0 U/L (OCA 10 mg), and −3.8 U/L, −10.6 U/L, and −12.2 U/L for AST, respectively.
Discussion: OCA significantly reduced ALT and AST as early as week 2 in both OCA treatment groups. By month 6, ALT normalized in > 50% and AST in nearly 33% of patients with elevated BL levels. These data suggest that OCA meaningfully reduces ALT and AST to levels indicative of improved clinical outcomes. Further research is needed to understand how measuring ALT and AST levels can enhance the assessment of PBC treatment response and minimize the risk of disease progression.
Disclosures:
Robert G. Gish, MD1, Darren Wheeler, PhD2, Jing Li, PhD2, Christopher Gasink, MD2, David W. Victor, MD3. P1754 - Effect of Obeticholic Acid on Reduction and Normalization of Alanine Aminotransferase and Aspartate Aminotransferase in the Phase 3 POISE Trial in Primary Biliary Cholangitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Robert G. Gish Consultants, LLC, San Diego, CA; 2Intercept Pharmaceuticals, Inc., Morristown, NJ; 3Houston Methodist Hospital, Houston, TX
Introduction: Obeticholic acid (OCA) received accelerated approval for primary biliary cholangitis (PBC) based on improvements in alkaline phosphatase (ALP) and total bilirubin levels in the phase 3 POISE study. Elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are linked to an increased risk of negative outcomes in patients with PBC. We evaluated the effect of OCA on ALT and AST, including the proportion of patients normalizing these levels (ALT ≤ 41 U/L and AST ≤ 34 U/L) from abnormal baseline (BL) values.
Methods: In POISE, PBC patients with inadequate response or intolerance to ursodeoxycholic acid were randomized 1:1:1 to receive placebo (PBO), OCA titration (OCA 5 mg with option to titrate to 10 mg at month 6), or OCA 10 mg for 12 months. The Cochran–Mantel–Haenszel method was used to assess the association between OCA treatment and ALT or AST normalization among patients with elevated BL levels.
Results: In the intention-to-treat (ITT) population, mean BL ALT values were 56.0 U/L for PBO, 61.6 U/L for OCA titration, and 56.3 U/L for OCA 10 mg. Baseline AST levels were 48.8, 52.3, and 50.5 U/L for PBO, OCA titration, and OCA 10 mg, respectively. Among patients with ALT > 41 U/L at BL (61%), 16% (7/45) on PBO normalized at month 6 vs 55% (26/47) on OCA 5 mg and 53% (21/40) on OCA 10 mg (p ≤ 0.001 for both; Fig. 1A). By 2 weeks, 3 to 4 times more patients in both OCA groups normalized ALT (both p< 0.01 vs PBO). Among patients with AST > 34 U/L at BL (71%), normalization rates at month 6 were 12% (6/51) on PBO, 30% (16/53) on OCA 5 mg, and 31% (15/49) on OCA 10 mg (p < 0.05 for both; Fig. 1B). The proportions of patients normalizing ALT and AST on OCA were similar at month 12. In the ITT population, ALT and AST reductions were significant for both OCA groups vs PBO at all visits (all p < 0.001) as early as week 2. Least-squares mean changes from BL for ALT were −6.6 U/L (PBO), −18.9 U/L (OCA 5 mg), and −22.0 U/L (OCA 10 mg), and −3.8 U/L, −10.6 U/L, and −12.2 U/L for AST, respectively.
Discussion: OCA significantly reduced ALT and AST as early as week 2 in both OCA treatment groups. By month 6, ALT normalized in > 50% and AST in nearly 33% of patients with elevated BL levels. These data suggest that OCA meaningfully reduces ALT and AST to levels indicative of improved clinical outcomes. Further research is needed to understand how measuring ALT and AST levels can enhance the assessment of PBC treatment response and minimize the risk of disease progression.

Figure: Figure 1. Proportion of patients treated with placebo, OCA 5 to 10 mg titration, or OCA 10 mg in the POISE study who achieved normalization of ALT (A) and AST (B) levels from baseline to Month 12.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; OCA, obeticholic acid.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; OCA, obeticholic acid.
Disclosures:
Robert Gish: Abbott – Advisor or Review Panel Member, Consultant. AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker’s contract for promotional talks. Altimmune – Advisor or Review Panel Member, Consultant. AngioCrine – Stock Options. Antios – Advisor or Review Panel Member, Consultant. Arrowhead – Advisor or Review Panel Member, Consultant. BMS – Speaker’s contract for promotional talks. CoCrystal – Minor stock shareholder (liver space noted only). CymaBay Therapeutics – Data safety monitoring board. Durect – Data safety monitoring board. Dynavax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Eiger – Advisor or Review Panel Member, Consultant, Stock Options. Eisai – Advisor or Review Panel Member, Consultant, Speaker’s contract for promotional talks. Enyo – Advisor or Review Panel Member, Consultant. Fujifilm/Wako – Advisory consultant, diagnostic companies. Genentech – Advisor or Review Panel Member, Consultant, Speaker’s contract for promotional talks. Genlantis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Stock Options. Gerson Lehrman Group – Advisor or Review Panel Member, Consultant. Gilead Sciences – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker’s contract for promotional talks. Helios – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. HepaTx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Stock Options. HepQuant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Stock Options. Intercept Pharmaceuticals Inc. – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker’s contract for promotional talks. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Merck – Advisor or Review Panel Member, Consultant. Perspectum – Advisory consultant, diagnostic companies. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Prodigy – Advisory Committee/Board Member, Chair of clinical advisory board. Quest – Advisory consultant, diagnostic companies:. RiboSciences – Minor stock shareholder (liver space noted only). Sonic Incytes – Advisory consultant, diagnostic companies:. Takeda – Data safety monitoring board. Topography Health – Advisor or Review Panel Member, Consultant, Current clinical trials alliance. Venatorx – Advisor or Review Panel Member, Consultant.
Darren Wheeler: Intercept Pharmaceuticals, Inc. – Employee.
Jing Li: Intercept Pharmaceuticals Inc. – Employee.
Christopher Gasink: Intercept Pharmaceuticals, Inc. – Employee. Johnson & Johnson – Employee.
David Victor: Gilead – speaker fees. Intercept Pharmaceuticals, Inc. – Advisory Committee/Board Member, Consultant, Speaker fees. Sebela – Consultant.
Robert G. Gish, MD1, Darren Wheeler, PhD2, Jing Li, PhD2, Christopher Gasink, MD2, David W. Victor, MD3. P1754 - Effect of Obeticholic Acid on Reduction and Normalization of Alanine Aminotransferase and Aspartate Aminotransferase in the Phase 3 POISE Trial in Primary Biliary Cholangitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
