Monday Poster Session
Category: Biliary/Pancreas
P1773 - Rituximab Therapy for Steroid Relapsing Autoimmune Pancreatitis: A Systematic Review and Meta-Analysis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- VB
Vinay C. Bellur, MBBS
Ramaiah Medical College
Bengaluru, Karnataka, India
Presenting Author(s)
Vinay C. Bellur, MBBS1, Ananya Prasad, MBBS2, Shreya Narayan, MBBS3, Ankita Raj, MBBS4, Deepak B Shivananda, MBBS3, Adithya Sathya narayana, MBBS1, Druvadeep Srinivas, MBBS5
1Ramaiah Medical College, Bengaluru, Karnataka, India; 2Ramaiah Medical College, Bangalore, Karnataka, India; 3Bangalore Medical College, Bangalore, Karnataka, India; 4Bangalore Medical College and Research Institute, Bangalore, Karnataka, India; 5Raja Rajeswari Medical College & Hospital, Bengaluru, Karnataka, India
Introduction: The first line of treatment for Autoimmune Pancreatitis(AIP) is Corticosteroids, which is associated with a relapse rate of 30-50%, especially in Type 1 AIP.Long-term steroid therapy is also linked to several adverse effects.Recently, rituximab(RTX), an antiCD20 monoclonal antibody,has proven to be an efficient addition for reducing the rate of relapse.Through this study,we aim to analyze the effectiveness of RTX in the management of steroid-relapsing AIP and include RTX as a stepping stone for further optimization of the treatment of AIP.
Methods: A systematic search was conducted in Pubmed,Google Scholar and Scopus.A boolean expression was constructed to search the databases.Clinical Trials and Observational studies assessing the efficacy (relapse rate in AIP) were selected for the Meta-Analysis.Raw data was extracted and analyzed in RStudio.The primary outcome was the relapse rates in patients of RTX Therapy and their 95% confidence intervals.The Random intercept logistic regression model and Clopper-Pearson confidence interval for individual studies were used.
We estimated the between-study variance(tau^2) using the restricted maximum-likelihood estimator and derived confidence intervals for tau^2 and tau using the Q-Profile method.
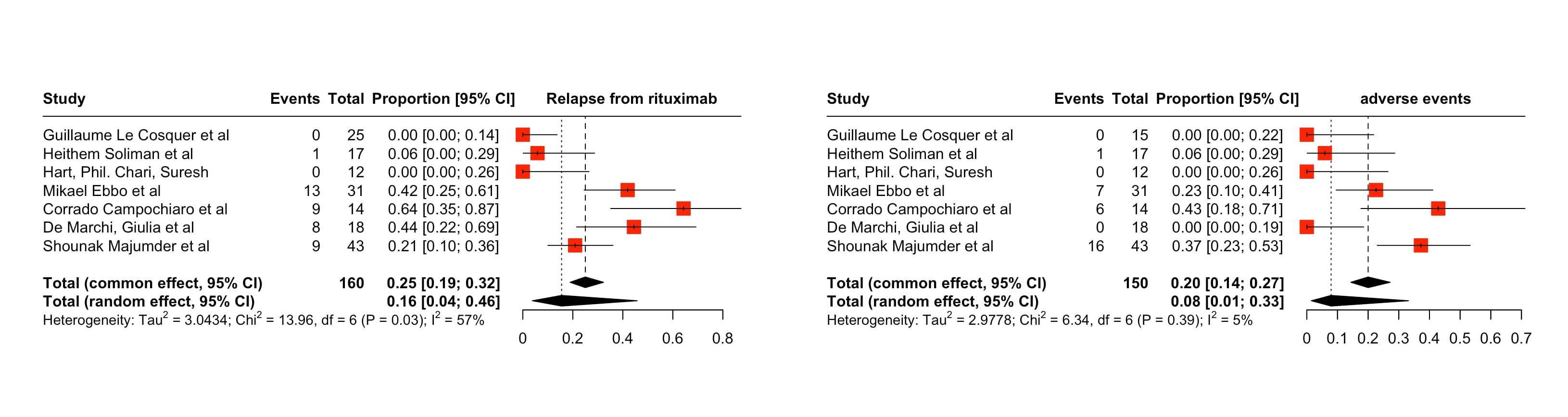
Results: Our meta-analysis included 7 studies with 160 observations and 40 events of relapse of AIP. The random effects model indicated an Event proportion of 0.1551 (95% CI:p=0.0001), reflecting substantial heterogeneity (tau^2=3.0434; tau=1.7445, 95% CI: I^2=57%, 95% CI: H=1.53 [1.00;2.32].The test of heterogeneity was significant.The Random effects model indicated that the relapse of AIP in RTX therapy was 0.1551 with 95% Confidence Interval. The second outcome which was adverse events related to RTX therapy was found to be 0.2000 [95% CI:0.1435;0.2717] with 30 outcomes of adverse events in the population of 150 from the common effects model which reflected substantial homogeneity of data (tau^2=2.9778;tau=1.7256 I^2=5.3% [0.0%;72.4%]; H=1.03 [1.00;1.90];95% CI)
Discussion: Our review showed that 15% of patients on Rituximab relapsed, which is significantly lower compared to the rates of relapse in steroid therapy ranging from 30-50%.Only 20% of patients undergoing Rituximab treatment had adverse events, which mainly involved hypersensitivity and infections indicating Rituximab should be used for streamlining the treatment of Steroid Relapsing Autoimmune Pancreatitis.
Disclosures:
Vinay C. Bellur, MBBS1, Ananya Prasad, MBBS2, Shreya Narayan, MBBS3, Ankita Raj, MBBS4, Deepak B Shivananda, MBBS3, Adithya Sathya narayana, MBBS1, Druvadeep Srinivas, MBBS5. P1773 - Rituximab Therapy for Steroid Relapsing Autoimmune Pancreatitis: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Ramaiah Medical College, Bengaluru, Karnataka, India; 2Ramaiah Medical College, Bangalore, Karnataka, India; 3Bangalore Medical College, Bangalore, Karnataka, India; 4Bangalore Medical College and Research Institute, Bangalore, Karnataka, India; 5Raja Rajeswari Medical College & Hospital, Bengaluru, Karnataka, India
Introduction: The first line of treatment for Autoimmune Pancreatitis(AIP) is Corticosteroids, which is associated with a relapse rate of 30-50%, especially in Type 1 AIP.Long-term steroid therapy is also linked to several adverse effects.Recently, rituximab(RTX), an antiCD20 monoclonal antibody,has proven to be an efficient addition for reducing the rate of relapse.Through this study,we aim to analyze the effectiveness of RTX in the management of steroid-relapsing AIP and include RTX as a stepping stone for further optimization of the treatment of AIP.
Methods: A systematic search was conducted in Pubmed,Google Scholar and Scopus.A boolean expression was constructed to search the databases.Clinical Trials and Observational studies assessing the efficacy (relapse rate in AIP) were selected for the Meta-Analysis.Raw data was extracted and analyzed in RStudio.The primary outcome was the relapse rates in patients of RTX Therapy and their 95% confidence intervals.The Random intercept logistic regression model and Clopper-Pearson confidence interval for individual studies were used.
We estimated the between-study variance(tau^2) using the restricted maximum-likelihood estimator and derived confidence intervals for tau^2 and tau using the Q-Profile method.
Results: Our meta-analysis included 7 studies with 160 observations and 40 events of relapse of AIP. The random effects model indicated an Event proportion of 0.1551 (95% CI:p=0.0001), reflecting substantial heterogeneity (tau^2=3.0434; tau=1.7445, 95% CI: I^2=57%, 95% CI: H=1.53 [1.00;2.32].The test of heterogeneity was significant.The Random effects model indicated that the relapse of AIP in RTX therapy was 0.1551 with 95% Confidence Interval. The second outcome which was adverse events related to RTX therapy was found to be 0.2000 [95% CI:0.1435;0.2717] with 30 outcomes of adverse events in the population of 150 from the common effects model which reflected substantial homogeneity of data (tau^2=2.9778;tau=1.7256 I^2=5.3% [0.0%;72.4%]; H=1.03 [1.00;1.90];95% CI)
Discussion: Our review showed that 15% of patients on Rituximab relapsed, which is significantly lower compared to the rates of relapse in steroid therapy ranging from 30-50%.Only 20% of patients undergoing Rituximab treatment had adverse events, which mainly involved hypersensitivity and infections indicating Rituximab should be used for streamlining the treatment of Steroid Relapsing Autoimmune Pancreatitis.

Figure: The images (right and left) depict forest plots indicating the overall proportion of relapse rates and adverse events in Rituximab therapy for Autoimmune Pancreatitis.
Disclosures:
Vinay Bellur indicated no relevant financial relationships.
Ananya Prasad indicated no relevant financial relationships.
Shreya Narayan indicated no relevant financial relationships.
Ankita Raj indicated no relevant financial relationships.
Deepak B Shivananda indicated no relevant financial relationships.
Adithya Sathya narayana indicated no relevant financial relationships.
Druvadeep Srinivas indicated no relevant financial relationships.
Vinay C. Bellur, MBBS1, Ananya Prasad, MBBS2, Shreya Narayan, MBBS3, Ankita Raj, MBBS4, Deepak B Shivananda, MBBS3, Adithya Sathya narayana, MBBS1, Druvadeep Srinivas, MBBS5. P1773 - Rituximab Therapy for Steroid Relapsing Autoimmune Pancreatitis: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
