Monday Poster Session
Category: Colorectal Cancer Prevention
P2122 - Non-Invasive Screening Tests for Colorectal Cancer: Analyzing the Performance of FIT and Multi-Target Stool DNA Test
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
.jpg)
Akash Patel, MD
Eisenhower Medical Center, Riverside Community Hospital
Palm Desert, CA
Presenting Author(s)
Akash Patel, MD1, Adewale Ajumobi, MD, FACG2
1Eisenhower Medical Center, Riverside Community Hospital, Palm Desert, CA; 2Eisenhower Health, Rancho Mirage, CA
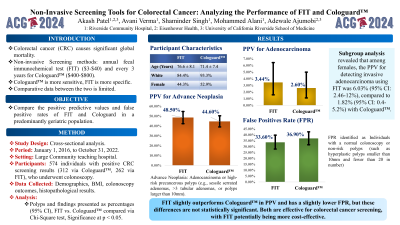
Introduction: Colorectal cancer (CRC) is a leading cause of cancer-related death. There are several screening options for CRC including annual fecal immunochemical test (FIT) and annual to every 3-year multitarget stool DNA-FIT (Cologuard™). There are few studies comparing FIT with Cologuard™ in a geriatric population. This study compares the performance (positive predictive value and false positive rates) of FIT and Cologuard™ in a geriatric population.
Methods: A cross-sectional study was conducted from January 1, 2016, to October 31, 2022, at a large community teaching hospital. Data included demographics, body mass index (BMI), colonoscopy findings, and histopathological results. Polyp characteristics were described as percentages within a 95% Confidence Interval (CI). Outcome differences were analyzed using the Chi-Square test, with significance set at p < 0.05.
Results: The study included 574 participants, with 312 positive Cologuard™ results and 262 positive FIT results. In the Cologuard™ group, 93.3% were white and 52.9% were female, with a mean age of 71.4 ± 7.4 years. In the FIT group, 84.4% were white and 44.3% were female, with a mean age of 76.6 ± 8.1 years. The positive predictive value (PPV) for invasive adenocarcinoma was 2.6% (95% CI: 1.11-4.99%) for Cologuard™ and 3.44% (95% CI: 1.58-6.42%) for FIT. For advanced neoplasia, the PPV was 44.60% (95% CI: 39-50.3%) for Cologuard™ and 48.50% (95% CI: 42.3-54.7%) for FIT. The false positive rate, identifying normal colonoscopy or no-risk polyps, was 36.9% (95% CI: 31.5-42.5%) for Cologuard™ and 33.6% (95% CI: 27.9-39.7%) for FIT. No significant differences were observed between FIT and Cologuard™ in false positivity rate, PPV for various findings, and subgroup analyses based on age, gender, and BMI.
Discussion: The PPV for invasive adenocarcinoma was slightly higher for FIT (3.44%) compared to Cologuard™ (2.6%). FIT also had a marginally higher PPV for advanced neoplasia (48.5% vs. 44.6%) and a slightly lower false positive rate (33.6% vs. 36.9%). These differences were not statistically significant. The findings suggest that both tests can be considered, with FIT being a more cost-effective option for CRC screening.
Disclosures:
Akash Patel, MD1, Adewale Ajumobi, MD, FACG2. P2122 - Non-Invasive Screening Tests for Colorectal Cancer: Analyzing the Performance of FIT and Multi-Target Stool DNA Test, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Eisenhower Medical Center, Riverside Community Hospital, Palm Desert, CA; 2Eisenhower Health, Rancho Mirage, CA
Introduction: Colorectal cancer (CRC) is a leading cause of cancer-related death. There are several screening options for CRC including annual fecal immunochemical test (FIT) and annual to every 3-year multitarget stool DNA-FIT (Cologuard™). There are few studies comparing FIT with Cologuard™ in a geriatric population. This study compares the performance (positive predictive value and false positive rates) of FIT and Cologuard™ in a geriatric population.
Methods: A cross-sectional study was conducted from January 1, 2016, to October 31, 2022, at a large community teaching hospital. Data included demographics, body mass index (BMI), colonoscopy findings, and histopathological results. Polyp characteristics were described as percentages within a 95% Confidence Interval (CI). Outcome differences were analyzed using the Chi-Square test, with significance set at p < 0.05.
Results: The study included 574 participants, with 312 positive Cologuard™ results and 262 positive FIT results. In the Cologuard™ group, 93.3% were white and 52.9% were female, with a mean age of 71.4 ± 7.4 years. In the FIT group, 84.4% were white and 44.3% were female, with a mean age of 76.6 ± 8.1 years. The positive predictive value (PPV) for invasive adenocarcinoma was 2.6% (95% CI: 1.11-4.99%) for Cologuard™ and 3.44% (95% CI: 1.58-6.42%) for FIT. For advanced neoplasia, the PPV was 44.60% (95% CI: 39-50.3%) for Cologuard™ and 48.50% (95% CI: 42.3-54.7%) for FIT. The false positive rate, identifying normal colonoscopy or no-risk polyps, was 36.9% (95% CI: 31.5-42.5%) for Cologuard™ and 33.6% (95% CI: 27.9-39.7%) for FIT. No significant differences were observed between FIT and Cologuard™ in false positivity rate, PPV for various findings, and subgroup analyses based on age, gender, and BMI.
Discussion: The PPV for invasive adenocarcinoma was slightly higher for FIT (3.44%) compared to Cologuard™ (2.6%). FIT also had a marginally higher PPV for advanced neoplasia (48.5% vs. 44.6%) and a slightly lower false positive rate (33.6% vs. 36.9%). These differences were not statistically significant. The findings suggest that both tests can be considered, with FIT being a more cost-effective option for CRC screening.
Disclosures:
Akash Patel indicated no relevant financial relationships.
Adewale Ajumobi indicated no relevant financial relationships.
Akash Patel, MD1, Adewale Ajumobi, MD, FACG2. P2122 - Non-Invasive Screening Tests for Colorectal Cancer: Analyzing the Performance of FIT and Multi-Target Stool DNA Test, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

