Monday Poster Session
Category: Colorectal Cancer Prevention
P2134 - Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA) Use Reduces Risk of Early-Onset Colorectal Cancer in Patients with Type 2 Diabetes-Mellitus (T2DM)
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Temitope Olasehinde, MD
University Hospitals Cleveland Medical Center, Case Western Reserve University
Cleveland, OH
Presenting Author(s)
Award: Presidential Poster Award
Temitope Olasehinde, MD1, Gregory Cooper, MD2, Jaime A. Perez, PhD3
1University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; 2Digestive Health Institute, University Hospitals Cleveland Medical Center, Cleveland, OH; 3University Hospitals Clinical Research Center, Cleveland, OH
Introduction: GLP-1RAs are currently utilized for the management of T2DM and obesity — two important risk factors for colorectal cancer development. Emerging literature has shown that GLP-1RAs are associated with a reduced risk of late-onset colorectal cancer (LO-CRC) in patients with T2DM. However, this effect has yet to be demonstrated for early-onset colorectal cancer (EO-CRC) — which has seen an alarming increase in incidence and has a greater association with T2DM and obesity compared to LO-CRC. We sought to examine the impact of GLP-1RA use on EO-CRC risk utilizing a large population-based database.
Methods: We performed a retrospective cohort study using a large federated de-identified health research network, TriNetX. We identified patients (age < 50 years) with a diagnosis of T2DM who were subsequently prescribed antidiabetic medications and had no prior CRC diagnosis. Within this population, we stratified patients into 2 cohorts based on first-time GLP-1RA use. We further performed subanalysis based on GLP-1RA use and the presence of obesity. We investigated the outcome of the first diagnosis of EO-CRC after GLP-1RA prescription. Propensity score matching was performed to balance cohorts. Differences between cohorts were calculated using t-test, chi-square/Fisher's exact test.
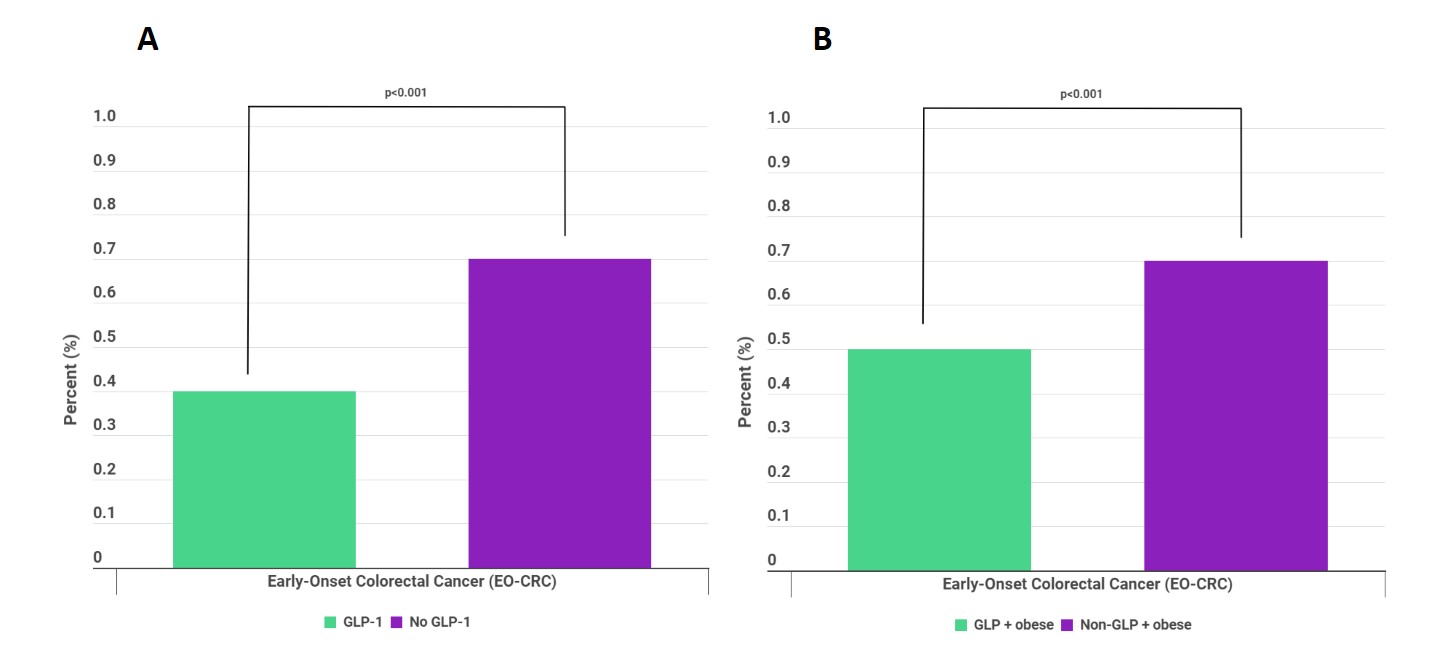
Results: We found a total of 1,815,788 patients with T2DM. Among them, 247,463 patients were in the GLP-1 cohort, whereas 1,568,325 were in the no GLP-1 cohort. After propensity score matching, both cohorts had 77,688 patients in each group. Compared to their no GLP-1 counterparts, the GLP-1 cohort was more likely to be older (44.21 ± 9.74 vs 36.45 ± 11.14, p< 0.001), female (56.3% vs 50.9%, p< 0.001), White (54.3% vs 53.4%, p< 0.001), non-Hispanic/Latino (65.1% vs 57.1%, p< 0.001), greater comorbidities (p< 0.001), and higher BMI (36.78 ± 7.03 vs 30.62 ± 8.48, p< 0.001) (Table 1). For the primary analysis, the GLP-1 cohort had significantly lower odds of developing EO-CRC (0.4% vs 0.7%, p< 0.001) compared to the no GLP-1 cohort (Figure 1A). In the subanalysis, the GLP+obese cohort had significantly lower odds of developing EO-CRC compared to the non-GLP+obese cohort (Figure 1B).
Discussion: This is the first large study to demonstrate that GLP-1RAs decrease the risk of developing EO-CRC in patients with T2DM regardless of weight. Future randomized controlled studies are needed to validate these findings, which may have significant implications for CRC prevention in younger patients.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Temitope Olasehinde, MD1, Gregory Cooper, MD2, Jaime A. Perez, PhD3. P2134 - Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA) Use Reduces Risk of Early-Onset Colorectal Cancer in Patients with Type 2 Diabetes-Mellitus (T2DM), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Temitope Olasehinde, MD1, Gregory Cooper, MD2, Jaime A. Perez, PhD3
1University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; 2Digestive Health Institute, University Hospitals Cleveland Medical Center, Cleveland, OH; 3University Hospitals Clinical Research Center, Cleveland, OH
Introduction: GLP-1RAs are currently utilized for the management of T2DM and obesity — two important risk factors for colorectal cancer development. Emerging literature has shown that GLP-1RAs are associated with a reduced risk of late-onset colorectal cancer (LO-CRC) in patients with T2DM. However, this effect has yet to be demonstrated for early-onset colorectal cancer (EO-CRC) — which has seen an alarming increase in incidence and has a greater association with T2DM and obesity compared to LO-CRC. We sought to examine the impact of GLP-1RA use on EO-CRC risk utilizing a large population-based database.
Methods: We performed a retrospective cohort study using a large federated de-identified health research network, TriNetX. We identified patients (age < 50 years) with a diagnosis of T2DM who were subsequently prescribed antidiabetic medications and had no prior CRC diagnosis. Within this population, we stratified patients into 2 cohorts based on first-time GLP-1RA use. We further performed subanalysis based on GLP-1RA use and the presence of obesity. We investigated the outcome of the first diagnosis of EO-CRC after GLP-1RA prescription. Propensity score matching was performed to balance cohorts. Differences between cohorts were calculated using t-test, chi-square/Fisher's exact test.
Results: We found a total of 1,815,788 patients with T2DM. Among them, 247,463 patients were in the GLP-1 cohort, whereas 1,568,325 were in the no GLP-1 cohort. After propensity score matching, both cohorts had 77,688 patients in each group. Compared to their no GLP-1 counterparts, the GLP-1 cohort was more likely to be older (44.21 ± 9.74 vs 36.45 ± 11.14, p< 0.001), female (56.3% vs 50.9%, p< 0.001), White (54.3% vs 53.4%, p< 0.001), non-Hispanic/Latino (65.1% vs 57.1%, p< 0.001), greater comorbidities (p< 0.001), and higher BMI (36.78 ± 7.03 vs 30.62 ± 8.48, p< 0.001) (Table 1). For the primary analysis, the GLP-1 cohort had significantly lower odds of developing EO-CRC (0.4% vs 0.7%, p< 0.001) compared to the no GLP-1 cohort (Figure 1A). In the subanalysis, the GLP+obese cohort had significantly lower odds of developing EO-CRC compared to the non-GLP+obese cohort (Figure 1B).
Discussion: This is the first large study to demonstrate that GLP-1RAs decrease the risk of developing EO-CRC in patients with T2DM regardless of weight. Future randomized controlled studies are needed to validate these findings, which may have significant implications for CRC prevention in younger patients.

Figure: Figure 1: A) Comparison of rates of early-onset colorectal cancer (EO-CRC) in patients on GLP-1RAs (GLP-1) vs patients not on GLP-1RAs (No GLP-1); B) Comparison of rates of EO-CRC in obese patients on GLP-1RAs (GLP + obese) vs obese patients not on GLP-1RAs (Non-GLP + obese)
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Temitope Olasehinde indicated no relevant financial relationships.
Gregory Cooper indicated no relevant financial relationships.
Jaime Perez indicated no relevant financial relationships.
Temitope Olasehinde, MD1, Gregory Cooper, MD2, Jaime A. Perez, PhD3. P2134 - Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA) Use Reduces Risk of Early-Onset Colorectal Cancer in Patients with Type 2 Diabetes-Mellitus (T2DM), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


