Monday Poster Session
Category: Colorectal Cancer Prevention
P2139 - Head-to-Head Real World Comparative Analysis of Two Artificial Intelligence-Assisted Colonoscopy Systems
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Tessa Herman, MD
University of Minnesota and Minneapolis VA Health Care System
Minneapolis, MN
Presenting Author(s)
Tessa Herman, MD1, Daniela Guerrero Vinsard, MD1, Morgan Freeman, MD2, Anders Westanmo, PharmD, MBA3, Amy Gravely, MA3, Mohammad Bilal, MD1, Brian Hanson, MD3
1University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN; 2University of Minnesota, Minneapolis, MN; 3Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Currently, there are multiple Food and Drug Administration (FDA)-approved/cleared artificial intelligence-assisted colonoscopy (AIAC) systems. Each system uses a unique proprietary algorithm derived from the dataset used to train the AI technology. An AI system with better performance directly impact patient outcomes. While there have been head-to-head comparisons of computer-aided diagnosis (CADx) systems to predict polyp histology, research lacks direct comparisons of FDA-approved computer-aided detection (CADe) technologies.
Methods: We performed a prospective comparative analysis of two CADe systems, Device A and Device B, in the first 6 months of their implementation at a Veterans Affairs Medical Center (May-October 2023). Two AIAC systems from each manufacturer were installed in 4 procedure rooms. Patients undergoing screening, surveillance, or diagnostic colonoscopy after positive fecal immunochemistry (FIT) were pseudorandomized to the CADe system based upon the next available room. The primary endpoint was adenoma detection rate (ADR). Secondary endpoints included sessile serrated lesions (SSL), advanced adenoma, hyperplastic, and benign, non-adenomatous, non-hyperplastic (BNANH) lesion detection rates. Statistical analysis was performed using Pearson’s chi-square or a two sample T-test, as applicable.
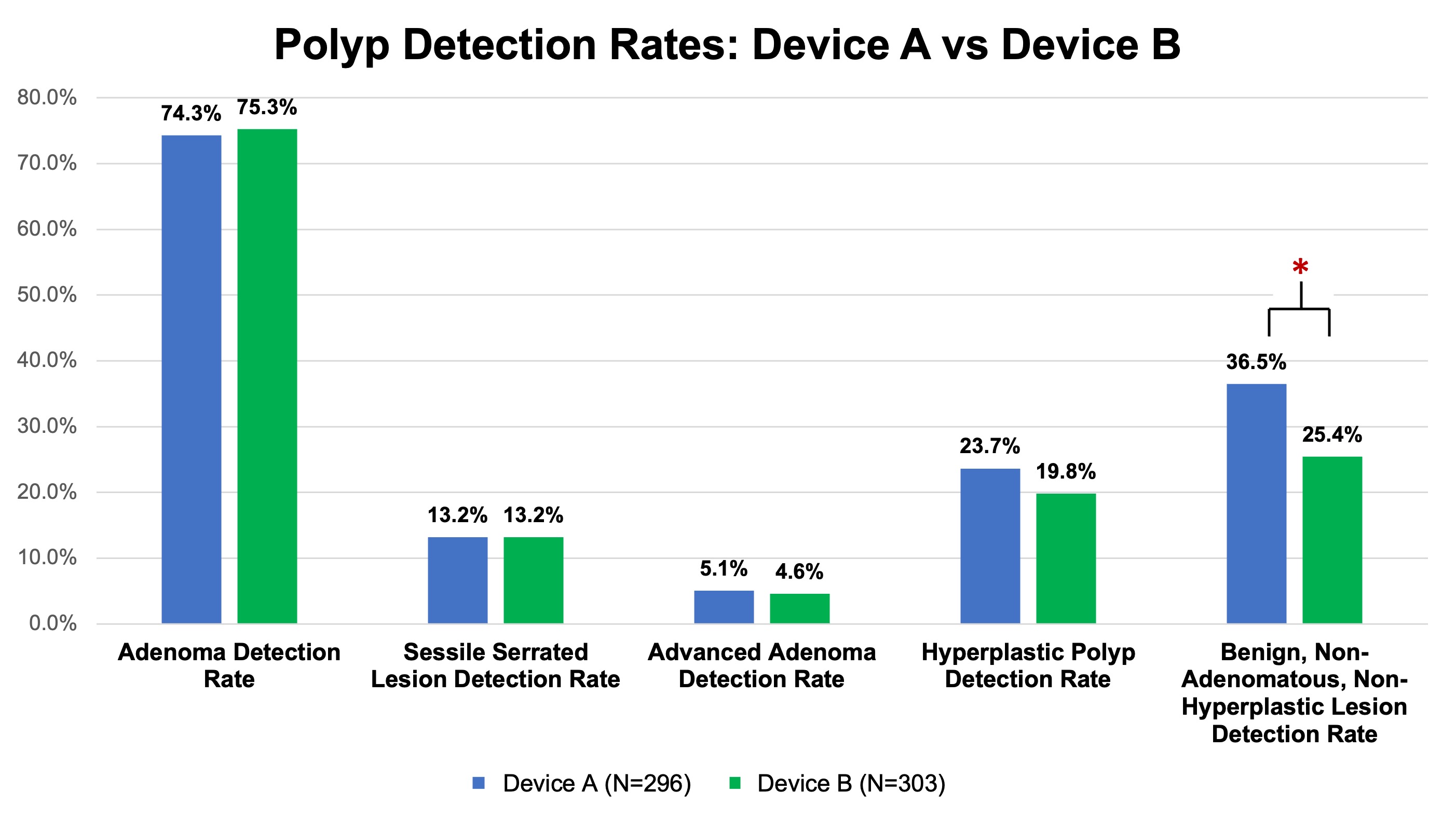
Results: 599 colonoscopies were completed: 296 with Device A and 303 with Device B. Patient and procedural demographics were similar among groups (Table 1). ADR was 74.3% with Device A versus 75.3% with Device B (p=0.79). BNANH detection rates were significantly higher with Device A (36.5% versus 25.4%, p=0.003). Advanced adenoma, SSL, and hyperplastic detection rates were similar (Figure 1). The mean number of polyps resected were similar (Device A mean 4.1, SD 3.6 versus Device B mean 3.8, SD 3.9; p=0.42). Withdrawal times were comparable.
Discussion: The AIAC devices performed similarly in detecting adenomas, demonstrating flexibility in between devices for the prevention of colorectal cancer. However, Device A saw significantly higher resection rates of BNANH lesions, which may have important clinical and financial implications. As additional CADe devices come to market and are implemented into gastroenterology practices, further head-to-head trials would be useful to guide device purchasing decisions and, most importantly, its impact on patient outcomes.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Tessa Herman, MD1, Daniela Guerrero Vinsard, MD1, Morgan Freeman, MD2, Anders Westanmo, PharmD, MBA3, Amy Gravely, MA3, Mohammad Bilal, MD1, Brian Hanson, MD3. P2139 - Head-to-Head Real World Comparative Analysis of Two Artificial Intelligence-Assisted Colonoscopy Systems, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN; 2University of Minnesota, Minneapolis, MN; 3Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Currently, there are multiple Food and Drug Administration (FDA)-approved/cleared artificial intelligence-assisted colonoscopy (AIAC) systems. Each system uses a unique proprietary algorithm derived from the dataset used to train the AI technology. An AI system with better performance directly impact patient outcomes. While there have been head-to-head comparisons of computer-aided diagnosis (CADx) systems to predict polyp histology, research lacks direct comparisons of FDA-approved computer-aided detection (CADe) technologies.
Methods: We performed a prospective comparative analysis of two CADe systems, Device A and Device B, in the first 6 months of their implementation at a Veterans Affairs Medical Center (May-October 2023). Two AIAC systems from each manufacturer were installed in 4 procedure rooms. Patients undergoing screening, surveillance, or diagnostic colonoscopy after positive fecal immunochemistry (FIT) were pseudorandomized to the CADe system based upon the next available room. The primary endpoint was adenoma detection rate (ADR). Secondary endpoints included sessile serrated lesions (SSL), advanced adenoma, hyperplastic, and benign, non-adenomatous, non-hyperplastic (BNANH) lesion detection rates. Statistical analysis was performed using Pearson’s chi-square or a two sample T-test, as applicable.
Results: 599 colonoscopies were completed: 296 with Device A and 303 with Device B. Patient and procedural demographics were similar among groups (Table 1). ADR was 74.3% with Device A versus 75.3% with Device B (p=0.79). BNANH detection rates were significantly higher with Device A (36.5% versus 25.4%, p=0.003). Advanced adenoma, SSL, and hyperplastic detection rates were similar (Figure 1). The mean number of polyps resected were similar (Device A mean 4.1, SD 3.6 versus Device B mean 3.8, SD 3.9; p=0.42). Withdrawal times were comparable.
Discussion: The AIAC devices performed similarly in detecting adenomas, demonstrating flexibility in between devices for the prevention of colorectal cancer. However, Device A saw significantly higher resection rates of BNANH lesions, which may have important clinical and financial implications. As additional CADe devices come to market and are implemented into gastroenterology practices, further head-to-head trials would be useful to guide device purchasing decisions and, most importantly, its impact on patient outcomes.

Figure: Polyp Resection Rates of Device A versus Device B – Device A and Device B had similar detection rates of most polyp types: adenoma detection rate (ADR), sessile serrated lesion detection rate, advanced ADR, and hyperplastic detection rate. There was a statistically higher rate of benign, non-adenomatous, non-hyperplastic pathology in Device A.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Tessa Herman indicated no relevant financial relationships.
Daniela Guerrero Vinsard indicated no relevant financial relationships.
Morgan Freeman indicated no relevant financial relationships.
Anders Westanmo indicated no relevant financial relationships.
Amy Gravely indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Speakers Bureau.
Brian Hanson: Motus GI – Consultant.
Tessa Herman, MD1, Daniela Guerrero Vinsard, MD1, Morgan Freeman, MD2, Anders Westanmo, PharmD, MBA3, Amy Gravely, MA3, Mohammad Bilal, MD1, Brian Hanson, MD3. P2139 - Head-to-Head Real World Comparative Analysis of Two Artificial Intelligence-Assisted Colonoscopy Systems, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
