Monday Poster Session
Category: Esophagus
P2208 - Assessing Risk of Gastroesophageal Reflux Disease and its Complications in Patients Starting Tirzepatide
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- BL
Benjamin Liu, MD
MetroHealth Medical Center
Cleveland, OH
Presenting Author(s)
Benjamin Liu, MD1, William Liu, 2, Yan Sun, MD3, Gengqing Song, MD, PhD4
1MetroHealth Medical Center, Cleveland, OH; 2MetroHealth, Cleveland, OH; 3Case Western Reserve University / MetroHealth, Cleveland, OH; 4Metrohealth Medical Center, Cleveland, OH
Introduction: We previously reported that glucagon-like peptide-1 receptor agonists (GLP-1 RA) increase the risk of gastroesophageal reflux disease (GERD) and its complications. Tirzepatide (TZ) is a recently approved combined GLP-1 RA and glucose-dependent insulinotropic polypeptide diabetes medication associated with superior type 2 diabetes mellitus (T2DM) and weight loss outcomes compared to traditional GLP-1 RAs. We decided to examine the risk of GERD and its complications associated with TZ.
Methods: We used the TriNetX platform to identify adult patients with T2DM who started either TZ or other second-line T2DM medications (OSLT2DM) after TZ’s market approval (May 2022). Patients must have started this medication for the first time ever, could not have exposure to comparison medications within a year, and could not have prior diagnoses of GERD. We excluded patients with esophageal dysmotility, connective tissue diseases, or major upper GI surgery. We performed 1:1 propensity score matching based on demographics and patient conditions, medications, or substance use that could affect diabetes medication choice and risk of GERD. We used Kaplan-Meier and Cox-Proportional Hazards Ratios to identify the risk of new GERD, non-erosive reflux disease (NERD), erosive reflux disease (ERD), and esophageal stricture up to 2 years following index event. Sub-analyses comparing TZ to GLP-1 RAs and GLP-1 RAs to OSLT2DM.
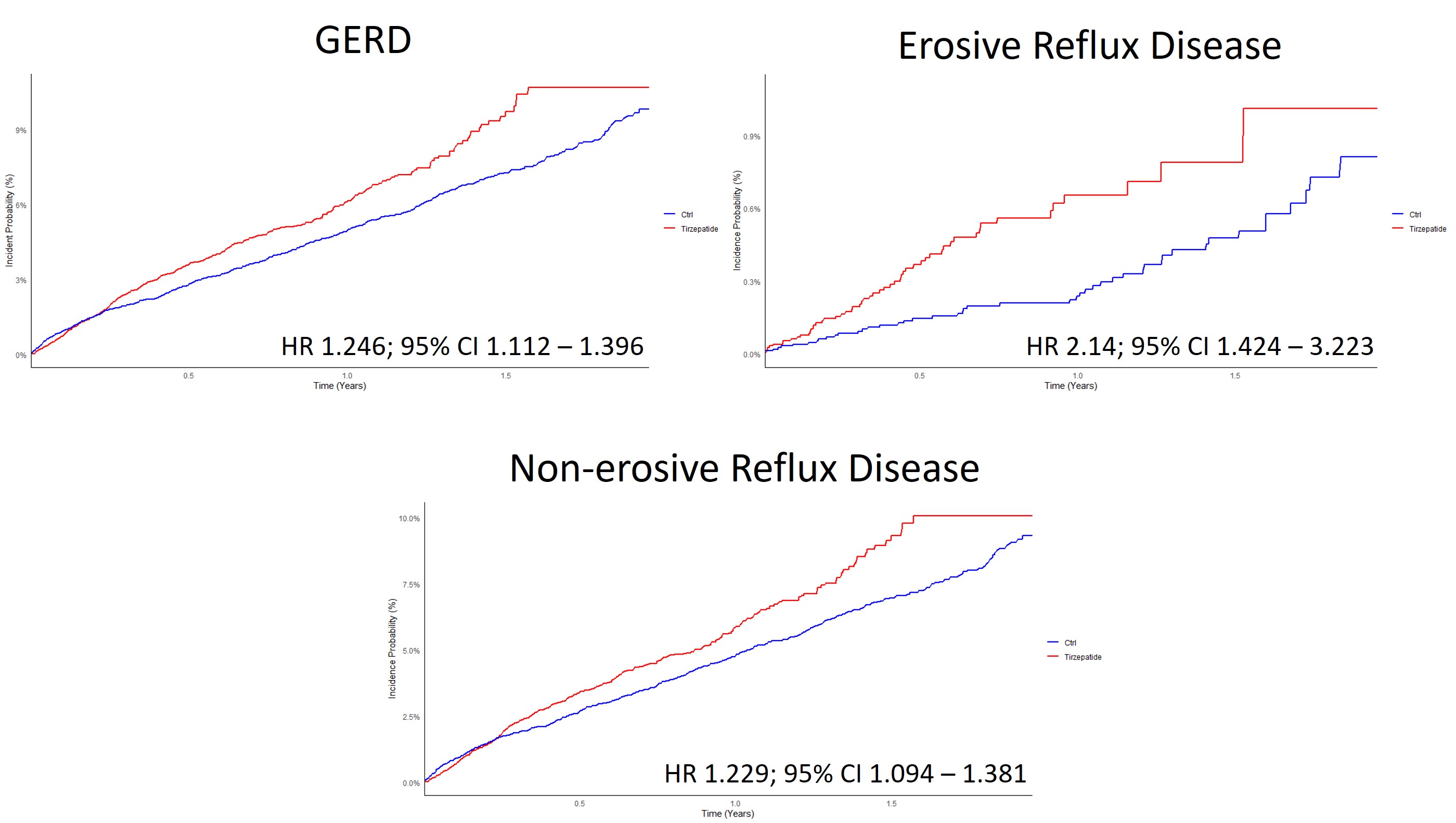
Results: A total 16,976 patients initiated TZ compared to 297,650 who started OSLT2DM. After matching, 16,805 patients were in each cohort. Mean follow-up was 180 days in the TZ cohort and 308 days in the OSLT2DM cohort. TZ patients developed new GERD at a rate of 67.04 events per 1000 person-years vs. 51.46 in the OSLT2DM cohort (HR 1.246; 95% CI 1.112 – 1.396. Patients on TZ developed ERD at a rate of 6.64 events per 1000 person-years vs. 3.32 for OSLT2DM (HR 2.14; 95% CI 1.424 – 3.223). Stricture events were too low to analyze. In the TZ vs. GLP-1 RA sub-analyses, no significant differences were observed. However, in the GLP-1 RA vs. OSLT2DM sub-analyses, GLP-1 RAs had a higher risk of GERD (HR 1.191; 95% CI 1.148 – 1.236), NERD (HR 1.199; 95% CI 1.154 – 1.246), and ERD (HR 1.186; 95% CI 1.048 – 1.342) compared to OSLT2DM.
Discussion: TZ appears to increase the risk of GERD and its short-term complications, aligning with prior data on GLP-1 RAs. Further research should confirm these findings prospectively and examine underlying mechanisms.
Disclosures:
Benjamin Liu, MD1, William Liu, 2, Yan Sun, MD3, Gengqing Song, MD, PhD4. P2208 - Assessing Risk of Gastroesophageal Reflux Disease and its Complications in Patients Starting Tirzepatide, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1MetroHealth Medical Center, Cleveland, OH; 2MetroHealth, Cleveland, OH; 3Case Western Reserve University / MetroHealth, Cleveland, OH; 4Metrohealth Medical Center, Cleveland, OH
Introduction: We previously reported that glucagon-like peptide-1 receptor agonists (GLP-1 RA) increase the risk of gastroesophageal reflux disease (GERD) and its complications. Tirzepatide (TZ) is a recently approved combined GLP-1 RA and glucose-dependent insulinotropic polypeptide diabetes medication associated with superior type 2 diabetes mellitus (T2DM) and weight loss outcomes compared to traditional GLP-1 RAs. We decided to examine the risk of GERD and its complications associated with TZ.
Methods: We used the TriNetX platform to identify adult patients with T2DM who started either TZ or other second-line T2DM medications (OSLT2DM) after TZ’s market approval (May 2022). Patients must have started this medication for the first time ever, could not have exposure to comparison medications within a year, and could not have prior diagnoses of GERD. We excluded patients with esophageal dysmotility, connective tissue diseases, or major upper GI surgery. We performed 1:1 propensity score matching based on demographics and patient conditions, medications, or substance use that could affect diabetes medication choice and risk of GERD. We used Kaplan-Meier and Cox-Proportional Hazards Ratios to identify the risk of new GERD, non-erosive reflux disease (NERD), erosive reflux disease (ERD), and esophageal stricture up to 2 years following index event. Sub-analyses comparing TZ to GLP-1 RAs and GLP-1 RAs to OSLT2DM.
Results: A total 16,976 patients initiated TZ compared to 297,650 who started OSLT2DM. After matching, 16,805 patients were in each cohort. Mean follow-up was 180 days in the TZ cohort and 308 days in the OSLT2DM cohort. TZ patients developed new GERD at a rate of 67.04 events per 1000 person-years vs. 51.46 in the OSLT2DM cohort (HR 1.246; 95% CI 1.112 – 1.396. Patients on TZ developed ERD at a rate of 6.64 events per 1000 person-years vs. 3.32 for OSLT2DM (HR 2.14; 95% CI 1.424 – 3.223). Stricture events were too low to analyze. In the TZ vs. GLP-1 RA sub-analyses, no significant differences were observed. However, in the GLP-1 RA vs. OSLT2DM sub-analyses, GLP-1 RAs had a higher risk of GERD (HR 1.191; 95% CI 1.148 – 1.236), NERD (HR 1.199; 95% CI 1.154 – 1.246), and ERD (HR 1.186; 95% CI 1.048 – 1.342) compared to OSLT2DM.
Discussion: TZ appears to increase the risk of GERD and its short-term complications, aligning with prior data on GLP-1 RAs. Further research should confirm these findings prospectively and examine underlying mechanisms.

Figure: Figure 1: Comparative Risk of Gastroesophageal Reflux Disease (GERD), Erosive Reflux Disease, and Non-erosive Reflux Disease up to Two Years Following Tirzepatide Administration.
Disclosures:
Benjamin Liu indicated no relevant financial relationships.
William Liu indicated no relevant financial relationships.
Yan Sun indicated no relevant financial relationships.
Gengqing Song indicated no relevant financial relationships.
Benjamin Liu, MD1, William Liu, 2, Yan Sun, MD3, Gengqing Song, MD, PhD4. P2208 - Assessing Risk of Gastroesophageal Reflux Disease and its Complications in Patients Starting Tirzepatide, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
