Monday Poster Session
Category: Esophagus
P2210 - Esophageal Motility Patterns in Patients Receiving GLP-1RA Therapy
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Annie L. Wang, BA, BS
Duke University School of Medicine
Durham, NC
Presenting Author(s)
Annie L. Wang, BA, BS1, David A. Leiman, MD, MSHP2
1Duke University School of Medicine, Durham, NC; 2Duke University School of Medicine, Chapel Hill, NC
Introduction: The use of glucagon-like peptide-1 receptor agonists (GLP-1RAs) is growing substantially. Given the impact GLP-1RAs can have on gastric emptying, several societies have provided recommendations for their use around endoscopic procedures to minimize complications. However, few data exist regarding the relationship between GLP-1RAs and esophageal function. This study aimed to describe esophageal motility patterns among patients using GLP-1RA medications undergoing high resolution manometry (HRM) and assess the impact specific medications have on motility.
Methods: All patients who underwent HRM between December 2022 to March 2024 were retrospectively evaluated for use of GLP-1RAs and were included independent of underlying comorbid conditions or HRM indication. Patients were excluded if GLP-1RA duration of use was < 5 weeks prior to HRM or if HRM was incomplete. Biodemographic data including age, sex, race, presence of diabetes, and most recent hemoglobin A1c were extracted from the electronic health record. Dose and type of GLP-1RA were recorded as were HRM diagnosis per Chicago Classification 4.0. Ineffective esophageal motility (IEM) and absent contractility diagnoses were grouped as hypomotility, while distal esophageal spasm and hypercontractile esophagus diagnoses were categorized as hypercontractility for analysis. Continuous variables were summarized, and Chi-square or Fisher’s exact tests were used as appropriate to compare those with a normal versus abnormal HRM and assess for differences in outcomes by GLP-1RA.
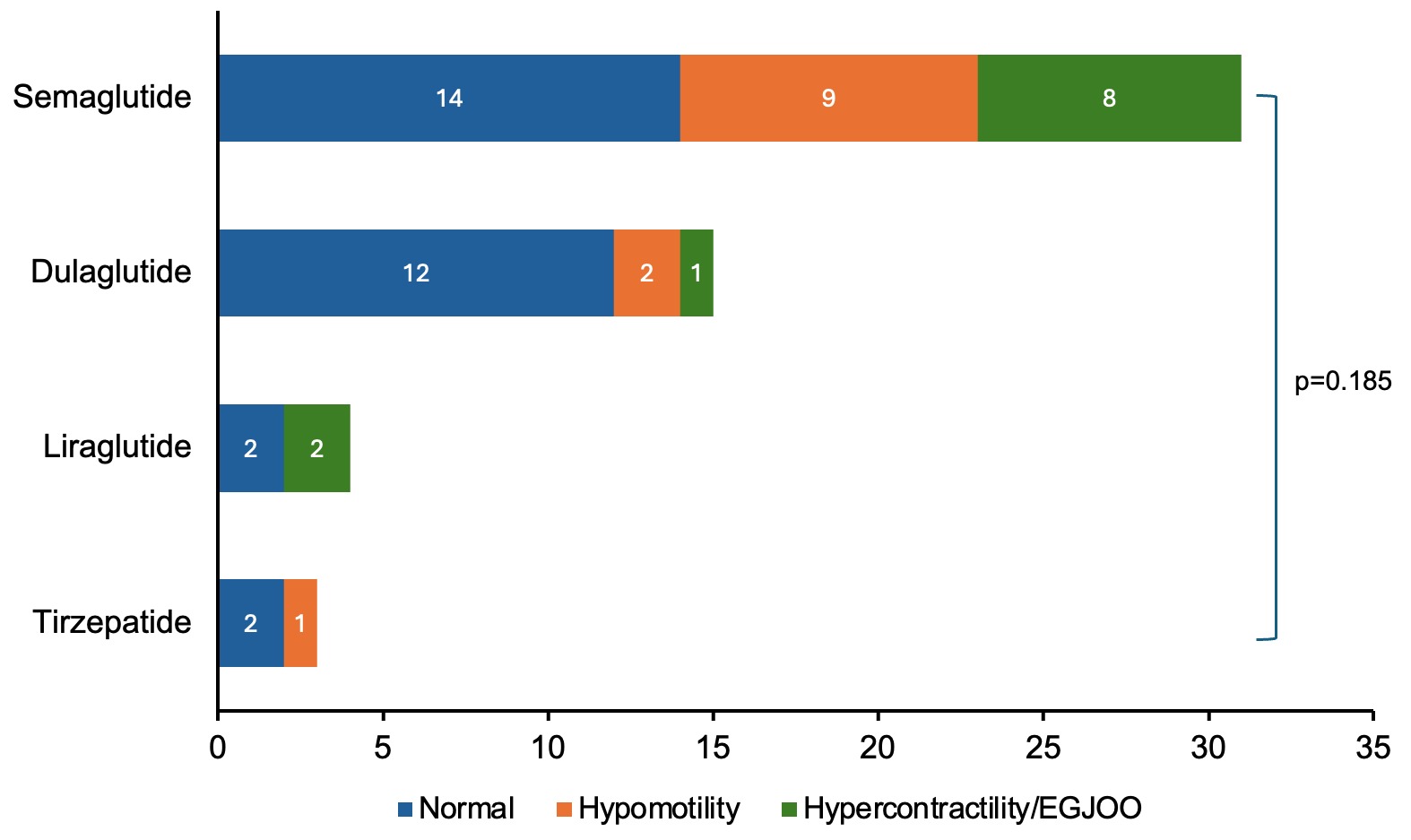
Results: Among 127 patients who underwent HRM during the study period, 53 met inclusion criteria (n=53, 69.8% female; median age 62 years). Most patients (n=30, 56.6%) had a normal HRM, whereas IEM was most common among those with abnormal findings, including 8 conclusive and 2 inconclusive diagnoses of IEM (Table 1, Figure 1). There was no significant association between specific GLP1-RA or dose and esophageal motility diagnosis. When comparing patients with a normal versus abnormal HRM, there was no significant association with GLP-1RA type, patient age, sex, BMI, presence of diabetes, or A1c.
Discussion: Despite their impact on delaying gastrointestinal motility, most patients on GLP-1RAs undergoing HRM had normal motility. There was no significant association between GLP-1RA type and esophageal motility.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Annie L. Wang, BA, BS1, David A. Leiman, MD, MSHP2. P2210 - Esophageal Motility Patterns in Patients Receiving GLP-1RA Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Duke University School of Medicine, Durham, NC; 2Duke University School of Medicine, Chapel Hill, NC
Introduction: The use of glucagon-like peptide-1 receptor agonists (GLP-1RAs) is growing substantially. Given the impact GLP-1RAs can have on gastric emptying, several societies have provided recommendations for their use around endoscopic procedures to minimize complications. However, few data exist regarding the relationship between GLP-1RAs and esophageal function. This study aimed to describe esophageal motility patterns among patients using GLP-1RA medications undergoing high resolution manometry (HRM) and assess the impact specific medications have on motility.
Methods: All patients who underwent HRM between December 2022 to March 2024 were retrospectively evaluated for use of GLP-1RAs and were included independent of underlying comorbid conditions or HRM indication. Patients were excluded if GLP-1RA duration of use was < 5 weeks prior to HRM or if HRM was incomplete. Biodemographic data including age, sex, race, presence of diabetes, and most recent hemoglobin A1c were extracted from the electronic health record. Dose and type of GLP-1RA were recorded as were HRM diagnosis per Chicago Classification 4.0. Ineffective esophageal motility (IEM) and absent contractility diagnoses were grouped as hypomotility, while distal esophageal spasm and hypercontractile esophagus diagnoses were categorized as hypercontractility for analysis. Continuous variables were summarized, and Chi-square or Fisher’s exact tests were used as appropriate to compare those with a normal versus abnormal HRM and assess for differences in outcomes by GLP-1RA.
Results: Among 127 patients who underwent HRM during the study period, 53 met inclusion criteria (n=53, 69.8% female; median age 62 years). Most patients (n=30, 56.6%) had a normal HRM, whereas IEM was most common among those with abnormal findings, including 8 conclusive and 2 inconclusive diagnoses of IEM (Table 1, Figure 1). There was no significant association between specific GLP1-RA or dose and esophageal motility diagnosis. When comparing patients with a normal versus abnormal HRM, there was no significant association with GLP-1RA type, patient age, sex, BMI, presence of diabetes, or A1c.
Discussion: Despite their impact on delaying gastrointestinal motility, most patients on GLP-1RAs undergoing HRM had normal motility. There was no significant association between GLP-1RA type and esophageal motility.

Figure: Figure 1: Variability in esophageal high resolution manometry (HRM) diagnoses per Chicago Classification 4.0 existed, but there was no significant association between glucagon-like peptide-1 receptor agonists (GLP-1RAs) and HRM diagnosis.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Annie Wang indicated no relevant financial relationships.
David Leiman: Astra Zeneca – Consultant. Bristol Myers Squibb – Stock-publicly held company(excluding mutual/index funds). Merck – Consultant. Novo Nordisk – Consultant. Regeneron – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member. TargetRWE – Advisor or Review Panel Member.
Annie L. Wang, BA, BS1, David A. Leiman, MD, MSHP2. P2210 - Esophageal Motility Patterns in Patients Receiving GLP-1RA Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
