Monday Poster Session
Category: Esophagus
P2212 - Balloon Cryotherapy is Highly Effective in Patients With Poor Response to Radiofrequency Ablation Therapy for Dysplastic Barrett’s Esophagus: Preliminary Results from the NO FEAR-BE Study
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Nicholas J. Shaheen, MD, MPH
University of North Carolina at Chapel Hill
Durham, NC
Presenting Author(s)
Nicholas J.. Shaheen, MD, MPH1, Marcia Canto, MD2, Cary C. Cotton, MD, MPH3, Prasad G. Iyer, MD, MS, FACG4, Julian Abrams, MD5, Harshit S.. Khara, MD, FACG6, Puja Elias, MD7, Amitabh Chak, MD8, Shervin Shafa, MD9, Shajan Peter, MD10, Nirav Thosani, MD11, Michael S.. Smith, MD, MBA12, Susan Moist, 13, Lindsay Cortright, 13, Kacie Walker, 13, Julia Kim, 13, Carson Jones, 13, Arvind Trindade, MD14
1University of North Carolina at Chapel Hill, Durham, NC; 2Johns Hopkins University School of Medicine, Baltimore, MD; 3University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; 4Mayo Clinic, Phoenix, AZ; 5Columbia University, New York, NY; 6Geisinger Medical Center, Danville, PA; 7Medical University of South Carolina, Charleston, SC; 8Digestive Health Institute, University Hospitals Cleveland Medical Center, Cleveland, OH; 9MedStar Georgetown University Hospital, Washington, DC; 10Basil I. Hirschowitz Endoscopic Center of Excellence, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 11McGovern Medical School at UTHealth, Houston, TX; 12Icahn School of Medicine at Mount Sinai, New York, NY; 13University of North Carolina at Chapel Hill, Chapel Hill, NC; 14Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ
Introduction: Radiofrequency ablation (RFA) is effective for the initial endoscopic eradication of Barrett’s esophagus (BE) with dysplasia, but up to a quarter of patients treated with RFA will not achieve complete eradication of intestinal metaplasia (CEIM). Data describing second-line treatments are sparse. We present preliminary results of balloon cryotherapy ablation (CbFAS) as rescue therapy after poor response to RFA.
Methods: Participants who had poor response to RFA, defined as persistent esophageal intestinal metaplasia or dysplasia after ≥3 RFA treatments or after two treatments with less than 50% resolution of baseline BE, were enrolled. Eligible participants received CbFAS every 3 months for up to 12 months or until CEIM was achieved. Participants who achieved CEIM were followed for an additional year. All cases which completed the treatment phase were analyzed. We estimated the cumulative incidence of CEIM, complete eradication of dysplasia (CED), and stricture, and calculated the exact binomial confidence limit for those proportions. A Poisson model of rates of recurrence of intestinal metaplasia and progression with 95% confidence limits was constructed.
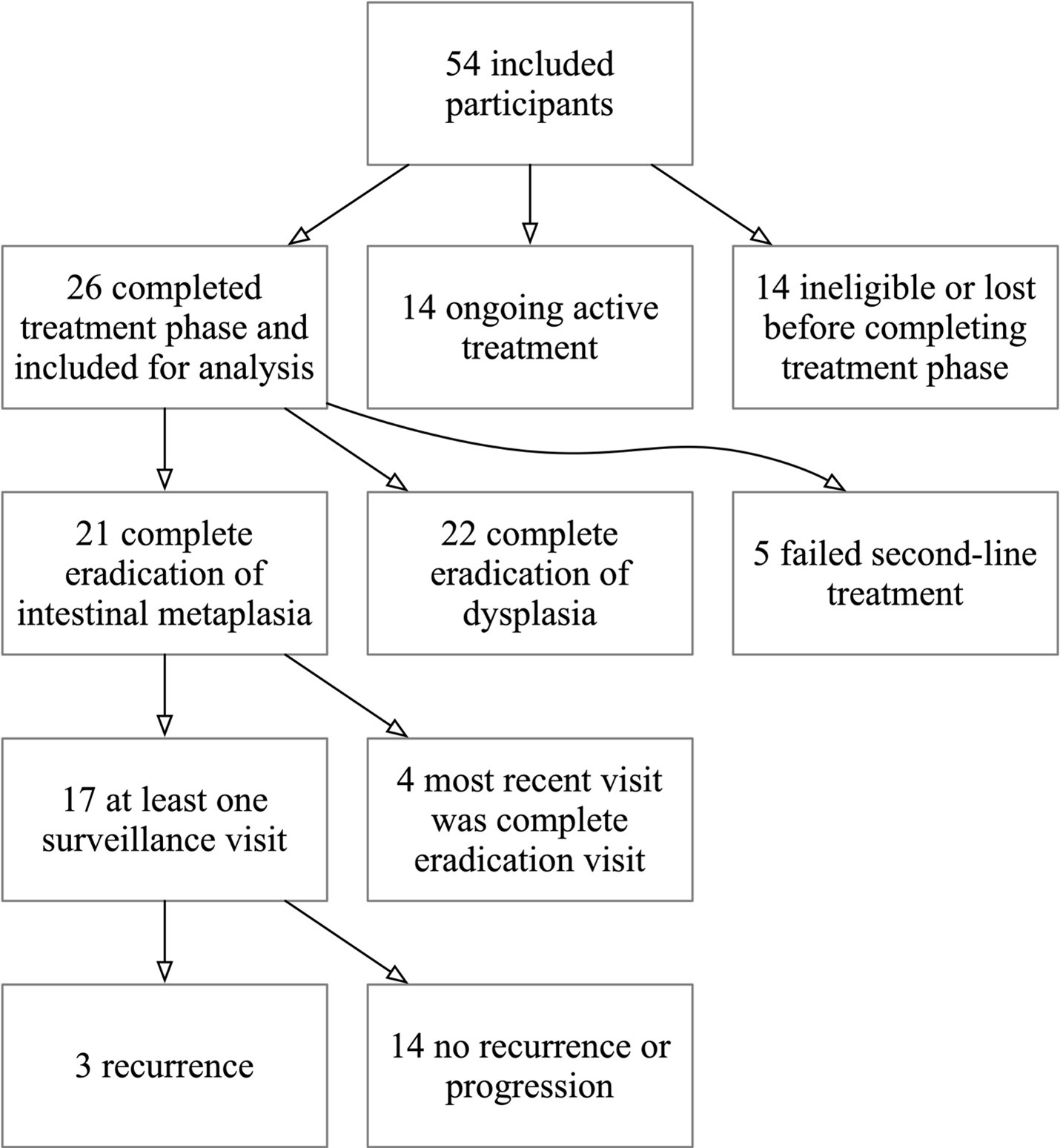
Results: Included in this preliminary analysis were 26 of 54 participants that completed the treatment phase (Table). Of these 26 participants, 21 (80.8%, 95% CI 60.6 - 93.4%) achieved CEIM at a mean 216.8 days and 3.2 CbFAS treatments. The same number achieved CED. Of 17 patients with at least one follow-up endoscopy after CEIM, there were 3 recurrences of intestinal metaplasia during a mean 288.0 days of follow-up after CEIM (0.22 per person-year, 95% CI 0.07 - 0.69). There were recurrences with definite dysplasia or progression (0.0%, 95% CI 0.0% - 19.5%). Of 26 participants, 2 (7.7%, 95% CI 0.9 - 25.1%) had a stricture during treatment or follow-up.
Discussion: In patients who were poorly responsive to RFA, CbFAS appears effective and durable in preliminary results. While patients who fail prior treatments are likely less treatment-responsive, CbFAS appeared similarly effective to established first-line treatments in treatment-naïve patients. Recurrence rates were comparable to rates reported in treatment-naïve patients in the first year. However, with only 17 participants in surveillance and < 1 year of follow up data on average, post-CEIM data were limited by statistical uncertainty.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Nicholas J.. Shaheen, MD, MPH1, Marcia Canto, MD2, Cary C. Cotton, MD, MPH3, Prasad G. Iyer, MD, MS, FACG4, Julian Abrams, MD5, Harshit S.. Khara, MD, FACG6, Puja Elias, MD7, Amitabh Chak, MD8, Shervin Shafa, MD9, Shajan Peter, MD10, Nirav Thosani, MD11, Michael S.. Smith, MD, MBA12, Susan Moist, 13, Lindsay Cortright, 13, Kacie Walker, 13, Julia Kim, 13, Carson Jones, 13, Arvind Trindade, MD14. P2212 - Balloon Cryotherapy is Highly Effective in Patients With Poor Response to Radiofrequency Ablation Therapy for Dysplastic Barrett’s Esophagus: Preliminary Results from the NO FEAR-BE Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of North Carolina at Chapel Hill, Durham, NC; 2Johns Hopkins University School of Medicine, Baltimore, MD; 3University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; 4Mayo Clinic, Phoenix, AZ; 5Columbia University, New York, NY; 6Geisinger Medical Center, Danville, PA; 7Medical University of South Carolina, Charleston, SC; 8Digestive Health Institute, University Hospitals Cleveland Medical Center, Cleveland, OH; 9MedStar Georgetown University Hospital, Washington, DC; 10Basil I. Hirschowitz Endoscopic Center of Excellence, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 11McGovern Medical School at UTHealth, Houston, TX; 12Icahn School of Medicine at Mount Sinai, New York, NY; 13University of North Carolina at Chapel Hill, Chapel Hill, NC; 14Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ
Introduction: Radiofrequency ablation (RFA) is effective for the initial endoscopic eradication of Barrett’s esophagus (BE) with dysplasia, but up to a quarter of patients treated with RFA will not achieve complete eradication of intestinal metaplasia (CEIM). Data describing second-line treatments are sparse. We present preliminary results of balloon cryotherapy ablation (CbFAS) as rescue therapy after poor response to RFA.
Methods: Participants who had poor response to RFA, defined as persistent esophageal intestinal metaplasia or dysplasia after ≥3 RFA treatments or after two treatments with less than 50% resolution of baseline BE, were enrolled. Eligible participants received CbFAS every 3 months for up to 12 months or until CEIM was achieved. Participants who achieved CEIM were followed for an additional year. All cases which completed the treatment phase were analyzed. We estimated the cumulative incidence of CEIM, complete eradication of dysplasia (CED), and stricture, and calculated the exact binomial confidence limit for those proportions. A Poisson model of rates of recurrence of intestinal metaplasia and progression with 95% confidence limits was constructed.
Results: Included in this preliminary analysis were 26 of 54 participants that completed the treatment phase (Table). Of these 26 participants, 21 (80.8%, 95% CI 60.6 - 93.4%) achieved CEIM at a mean 216.8 days and 3.2 CbFAS treatments. The same number achieved CED. Of 17 patients with at least one follow-up endoscopy after CEIM, there were 3 recurrences of intestinal metaplasia during a mean 288.0 days of follow-up after CEIM (0.22 per person-year, 95% CI 0.07 - 0.69). There were recurrences with definite dysplasia or progression (0.0%, 95% CI 0.0% - 19.5%). Of 26 participants, 2 (7.7%, 95% CI 0.9 - 25.1%) had a stricture during treatment or follow-up.
Discussion: In patients who were poorly responsive to RFA, CbFAS appears effective and durable in preliminary results. While patients who fail prior treatments are likely less treatment-responsive, CbFAS appeared similarly effective to established first-line treatments in treatment-naïve patients. Recurrence rates were comparable to rates reported in treatment-naïve patients in the first year. However, with only 17 participants in surveillance and < 1 year of follow up data on average, post-CEIM data were limited by statistical uncertainty.

Figure: Flow diagram showing inclusions and exclusion of participants as well as preliminary primary outcomes among 54 eligible NO-FEAR participants.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Nicholas Shaheen: Castle Biosciences – Consultant. CDx Medical – Grant/Research Support. Exact Sciences – Grant/Research Support. Fractyl – Grant/Research Support. GIE Medical – Grant/Research Support. Lucid – Grant/Research Support. Pentax – Grant/Research Support. Phathom – Consultant. Steris – Grant/Research Support.
Marcia Canto: Pentax – Grant/Research Support.
Cary Cotton: Pentax – Grant/Research Support.
Prasad G. Iyer: Castle Biosciences – Consultant, Grant/Research Support. CDx Medical – Consultant, Grant/Research Support. Exact Sciences – Advisory Committee/Board Member, Consultant, Grant/Research Support, Intellectual Property/Patents. Medtronic – Consultant. Pentax Medical – Consultant, Grant/Research Support.
Julian Abrams: Pentax – Grant/Research Support.
Harshit Khara: Boston Scientific – Consultant. Castle Biosciences – Consultant. ConMed – Consultant. Cook Medical – Consultant. Medtronic – Consultant. Neptune Medical – Consultant. Olympus America – Consultant. Pentax – Consultant.
Puja Elias: Pentax – Grant/Research Support.
Amitabh Chak: Lucid Diagnostics – Consultant, Grant/Research Support, Intellectual Property/Patents, Royalties, Stock Options, Stock-publicly held company(excluding mutual/index funds). MicroTech – Consultant. Steris – Consultant.
Shervin Shafa indicated no relevant financial relationships.
Shajan Peter: Olympus – Consultant.
Nirav Thosani indicated no relevant financial relationships.
Michael Smith: Castle Biosciences – Advisory Committee/Board Member, Consultant. CDx Diagnostics – Consultant. Lucid Diagnostics – Advisory Committee/Board Member, Consultant. Provation Medical – Consultant. Steris Endoscopy – Advisory Committee/Board Member, Consultant.
Susan Moist indicated no relevant financial relationships.
Lindsay Cortright indicated no relevant financial relationships.
Kacie Walker indicated no relevant financial relationships.
Julia Kim indicated no relevant financial relationships.
Carson Jones indicated no relevant financial relationships.
Arvind Trindade: Pentax – Grant/Research Support.
Nicholas J.. Shaheen, MD, MPH1, Marcia Canto, MD2, Cary C. Cotton, MD, MPH3, Prasad G. Iyer, MD, MS, FACG4, Julian Abrams, MD5, Harshit S.. Khara, MD, FACG6, Puja Elias, MD7, Amitabh Chak, MD8, Shervin Shafa, MD9, Shajan Peter, MD10, Nirav Thosani, MD11, Michael S.. Smith, MD, MBA12, Susan Moist, 13, Lindsay Cortright, 13, Kacie Walker, 13, Julia Kim, 13, Carson Jones, 13, Arvind Trindade, MD14. P2212 - Balloon Cryotherapy is Highly Effective in Patients With Poor Response to Radiofrequency Ablation Therapy for Dysplastic Barrett’s Esophagus: Preliminary Results from the NO FEAR-BE Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
