Monday Poster Session
Category: Esophagus
P2327 - Dupilumab in Combination Biologic Therapy in a Patient With Eosinophilic Esophagitis and Undifferentiated Connective Tissue Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
Guoyi Lai, BA, BS, MS
Duke University School of Medicine
Durham, NC
Presenting Author(s)
Guoyi Lai, BA, BS, MS1, David A. Leiman, MD, MSHP2
1Duke University School of Medicine, Durham, NC; 2Duke University School of Medicine, Chapel Hill, NC
Introduction: Eosinophilic esophagitis (EoE) is a chronic allergic inflammatory condition with a rising prevalence and has been associated with autoimmune conditions, including connective tissue disorders. Dupilumab is a humanized monoclonal antibody approved for treatment of EoE. Although it has been used in the management of other atopic conditions, few data exist on the use of dupilumab in combination with other biologic agents. We report a case of successful EoE management with dupilumab in a patient with co-existing undifferentiated connective tissue disease (UCTD) on concomitant adalimumab.
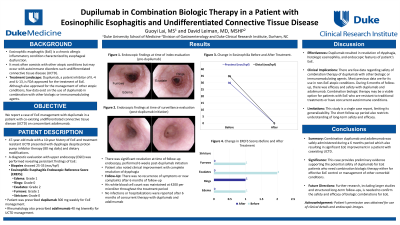
Case Description/Methods: A 47-year-old man with UCTD and EoE presented with dysphagia. He was diagnosed with EoE 10 years prior to presentation and previously had lack of clinical or histologic response to 80mg daily proton pump inhibitors (PPIs) or structured dietary elimination of dairy and gluten. An upper endoscopy (EGD) performed off of therapy revealed proximal and distal eosinophilia (25-35 eos/hpf), and graded using Eosinophilic Esophagitis Endoscopic Reference Score (EREFS) showed: Edema Grade 1, Rings Grade 0, Exudates Grade 2, Furrows Grade 1, and Stricture Grade 0. He was unable to obtain topical corticosteroids therefore dupilumab 300mg weekly was prescribed. He was concurrently placed on adalimubab 40mg every 14 days for his UCTD by rheumatology. Due to scheduling preferences of the patient, a surveillance EGD was performed 6 weeks after starting dupilumab and revealed resolution of eosinophilia (< 5 eso/hpf for distal, 0 eso/hpf for proximal; EREFS: G0R1Ex0F0S0) and clinical absence of dysphagia. A complete blood count was performed and the total white blood cell count was 4200 per microliter and there have been no reported infections or hospitalizations in 6 months of therapy.
Discussion: Dupilumab is a biologic treatment option increasingly used in EoE. Few data regarding the safety of combination biologic therapy with dupilumab exist and are primarily in the context of other atopic conditions. We present a novel description of dual biologic therapy with dupilumab and adalimumab for complex, overlapping conditions. We report successful treatment of EoE without associated infectious side-effects. In this case, combination therapy appears well-tolerated and clinically effective. Further information on the long-term safety of combination therapy, particularly among patients with EoE, is needed.
Disclosures:
Guoyi Lai, BA, BS, MS1, David A. Leiman, MD, MSHP2. P2327 - Dupilumab in Combination Biologic Therapy in a Patient With Eosinophilic Esophagitis and Undifferentiated Connective Tissue Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Duke University School of Medicine, Durham, NC; 2Duke University School of Medicine, Chapel Hill, NC
Introduction: Eosinophilic esophagitis (EoE) is a chronic allergic inflammatory condition with a rising prevalence and has been associated with autoimmune conditions, including connective tissue disorders. Dupilumab is a humanized monoclonal antibody approved for treatment of EoE. Although it has been used in the management of other atopic conditions, few data exist on the use of dupilumab in combination with other biologic agents. We report a case of successful EoE management with dupilumab in a patient with co-existing undifferentiated connective tissue disease (UCTD) on concomitant adalimumab.
Case Description/Methods: A 47-year-old man with UCTD and EoE presented with dysphagia. He was diagnosed with EoE 10 years prior to presentation and previously had lack of clinical or histologic response to 80mg daily proton pump inhibitors (PPIs) or structured dietary elimination of dairy and gluten. An upper endoscopy (EGD) performed off of therapy revealed proximal and distal eosinophilia (25-35 eos/hpf), and graded using Eosinophilic Esophagitis Endoscopic Reference Score (EREFS) showed: Edema Grade 1, Rings Grade 0, Exudates Grade 2, Furrows Grade 1, and Stricture Grade 0. He was unable to obtain topical corticosteroids therefore dupilumab 300mg weekly was prescribed. He was concurrently placed on adalimubab 40mg every 14 days for his UCTD by rheumatology. Due to scheduling preferences of the patient, a surveillance EGD was performed 6 weeks after starting dupilumab and revealed resolution of eosinophilia (< 5 eso/hpf for distal, 0 eso/hpf for proximal; EREFS: G0R1Ex0F0S0) and clinical absence of dysphagia. A complete blood count was performed and the total white blood cell count was 4200 per microliter and there have been no reported infections or hospitalizations in 6 months of therapy.
Discussion: Dupilumab is a biologic treatment option increasingly used in EoE. Few data regarding the safety of combination biologic therapy with dupilumab exist and are primarily in the context of other atopic conditions. We present a novel description of dual biologic therapy with dupilumab and adalimumab for complex, overlapping conditions. We report successful treatment of EoE without associated infectious side-effects. In this case, combination therapy appears well-tolerated and clinically effective. Further information on the long-term safety of combination therapy, particularly among patients with EoE, is needed.
Disclosures:
Guoyi Lai indicated no relevant financial relationships.
David Leiman: Astra Zeneca – Consultant. Bristol Myers Squibb – Stock-publicly held company(excluding mutual/index funds). Merck – Consultant. Novo Nordisk – Consultant. Regeneron – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member. TargetRWE – Advisor or Review Panel Member.
Guoyi Lai, BA, BS, MS1, David A. Leiman, MD, MSHP2. P2327 - Dupilumab in Combination Biologic Therapy in a Patient With Eosinophilic Esophagitis and Undifferentiated Connective Tissue Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
