Monday Poster Session
Category: Functional Bowel Disease
P2362 - A Multicenter, Sham-Controlled, Randomized Controlled Trial of Interferential Therapy for the Treatment of Chronic Constipation
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- JC
Jenna Clukey, BA
Center for Neurointestinal Health, Massachusetts General Hospital
Boston, MA
Presenting Author(s)
Award: Presidential Poster Award
Jenna Clukey, BA1, Joseph J.. Locascio, PhD2, Jack Semler, PhD3, Yogi Patel, PhD4, James Makous, PhD5, Ashok Attaluri, MD6, Braden Kuo, MD, MSc7, Kyle Staller, MD, MPH1
1Center for Neurointestinal Health, Massachusetts General Hospital, Boston, MA; 2Harvard Medical School, Arlington, MA; 3Independent Consultant, Amherst, NY; 4ReStalsis Health, Inc, Atlanta, GA; 5Makous Research, LLC, Carlsbad, CA; 6Olathe Medical Center, Olathe, KS; 7Massachusetts General Hospital, Boston, MA
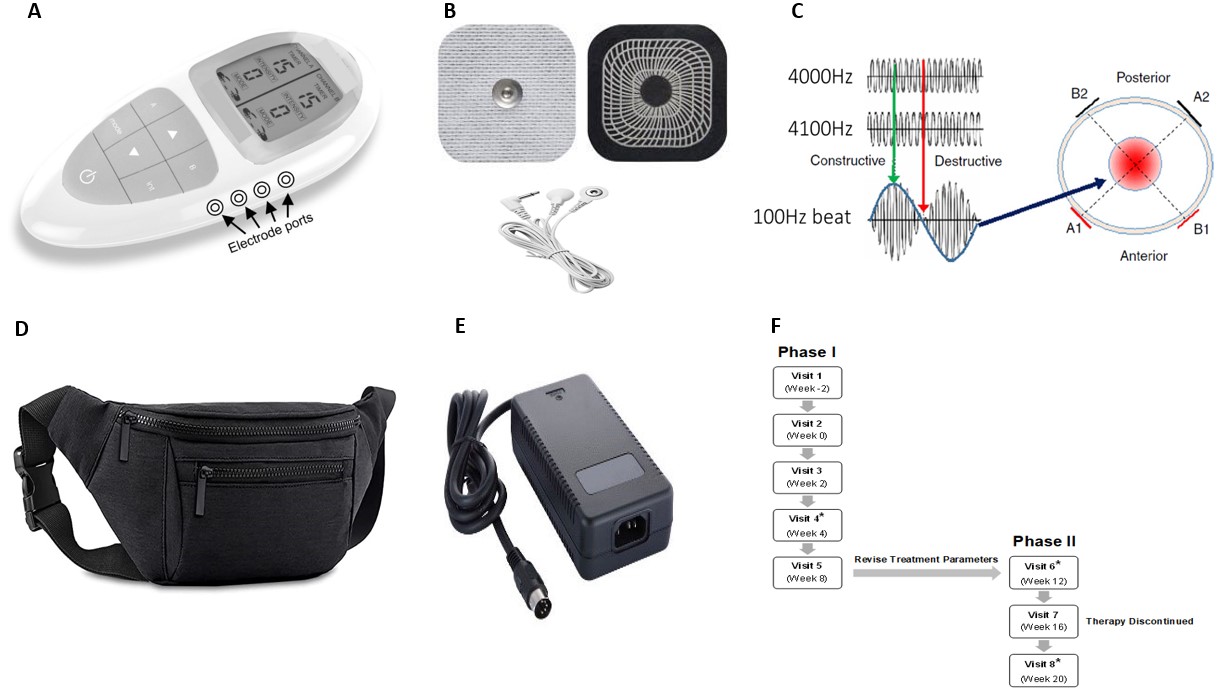
Introduction: Subjects with refractory chronic constipation (CC) have limited treatment options. Interferential Therapy (IFT, ReStalsis Health Inc.) delivers 2 out of phase sinusoidal currents transcutaneously from an external generator, which intersect intra-abdominally to produce a therapeutic current in the colon (Figure 1). We performed a pilot randomized controlled trial (RCT) to evaluate the efficacy and safety of IFT for the treatment of CC.
Methods: We performed a multicenter, double-blind, sham-controlled RCT (2:1, treatment:sham) trial of IFT in patients meeting Rome IV criteria for refractory CC (failed over the counter treatments for at least 3 months), with ≥1 spontaneous bowel movement (SBM)/week and < 3 SBMs/week. Patients recorded SBMs and complete SBMs (CSBMs)/week via a daily stool diary which patients started during a 2-week screening period followed by IFT or sham (in which the sinusoids did not intersect in the colon) treatment. Both groups completed treatment for an hour a day for 8 weeks. Then the treatment group transitioned into a maintenance phase where the device was used 3 days a week and the sham group received the IFT device for 8 weeks. Participants completed the Patient Assessment of Constipation Symptoms and the Patient Assessment of Constipation Quality of Life at weeks 0, 8, 16, and 20. Longitudinal, mixed effects between and within subject models were used for the analyses.<span style="mso-spacerun: yes;"> </span>
Results: We enrolled 17 patients (82% female) at 4 sites, 12 into the treatment group and 5 into sham therapy. The treatment group experienced significant increases in SBMs and CSBMs after starting IFT therapy (p = 0.03) (Table 1). There was a significant decrease in symptom severity, relative to baseline, in both the treatment and maintenance phases (-0.94, p=0.0002 for treatment; -0.97, p=0.0027 for maintenance)(Table 1) that was not seen in sham (p=0.075). Patient Assessment of Constipation Quality of Life improved in IFT treatment and maintenance therapies relative to baseline (-0.96, p< 0.0001; -0.81, p=0.0041) as well as for the sham group (p= 0.0131). Relative to sham, however, there were no significant differences in any outcomes.
Discussion: In this pilot RCT of IFT, SBMs and CSBMs increased with IFT use, but not significantly so over sham treatment. Numeric improvements without statistical differences underscores potential issues related to statistical power. Larger, sham-controlled trials are needed to determine the role of IFT in patients with CC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jenna Clukey, BA1, Joseph J.. Locascio, PhD2, Jack Semler, PhD3, Yogi Patel, PhD4, James Makous, PhD5, Ashok Attaluri, MD6, Braden Kuo, MD, MSc7, Kyle Staller, MD, MPH1. P2362 - A Multicenter, Sham-Controlled, Randomized Controlled Trial of Interferential Therapy for the Treatment of Chronic Constipation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Jenna Clukey, BA1, Joseph J.. Locascio, PhD2, Jack Semler, PhD3, Yogi Patel, PhD4, James Makous, PhD5, Ashok Attaluri, MD6, Braden Kuo, MD, MSc7, Kyle Staller, MD, MPH1
1Center for Neurointestinal Health, Massachusetts General Hospital, Boston, MA; 2Harvard Medical School, Arlington, MA; 3Independent Consultant, Amherst, NY; 4ReStalsis Health, Inc, Atlanta, GA; 5Makous Research, LLC, Carlsbad, CA; 6Olathe Medical Center, Olathe, KS; 7Massachusetts General Hospital, Boston, MA
Introduction: Subjects with refractory chronic constipation (CC) have limited treatment options. Interferential Therapy (IFT, ReStalsis Health Inc.) delivers 2 out of phase sinusoidal currents transcutaneously from an external generator, which intersect intra-abdominally to produce a therapeutic current in the colon (Figure 1). We performed a pilot randomized controlled trial (RCT) to evaluate the efficacy and safety of IFT for the treatment of CC.
Methods: We performed a multicenter, double-blind, sham-controlled RCT (2:1, treatment:sham) trial of IFT in patients meeting Rome IV criteria for refractory CC (failed over the counter treatments for at least 3 months), with ≥1 spontaneous bowel movement (SBM)/week and < 3 SBMs/week. Patients recorded SBMs and complete SBMs (CSBMs)/week via a daily stool diary which patients started during a 2-week screening period followed by IFT or sham (in which the sinusoids did not intersect in the colon) treatment. Both groups completed treatment for an hour a day for 8 weeks. Then the treatment group transitioned into a maintenance phase where the device was used 3 days a week and the sham group received the IFT device for 8 weeks. Participants completed the Patient Assessment of Constipation Symptoms and the Patient Assessment of Constipation Quality of Life at weeks 0, 8, 16, and 20. Longitudinal, mixed effects between and within subject models were used for the analyses.<span style="mso-spacerun: yes;"> </span>
Results: We enrolled 17 patients (82% female) at 4 sites, 12 into the treatment group and 5 into sham therapy. The treatment group experienced significant increases in SBMs and CSBMs after starting IFT therapy (p = 0.03) (Table 1). There was a significant decrease in symptom severity, relative to baseline, in both the treatment and maintenance phases (-0.94, p=0.0002 for treatment; -0.97, p=0.0027 for maintenance)(Table 1) that was not seen in sham (p=0.075). Patient Assessment of Constipation Quality of Life improved in IFT treatment and maintenance therapies relative to baseline (-0.96, p< 0.0001; -0.81, p=0.0041) as well as for the sham group (p= 0.0131). Relative to sham, however, there were no significant differences in any outcomes.
Discussion: In this pilot RCT of IFT, SBMs and CSBMs increased with IFT use, but not significantly so over sham treatment. Numeric improvements without statistical differences underscores potential issues related to statistical power. Larger, sham-controlled trials are needed to determine the role of IFT in patients with CC.

Figure: Figure 1A-F. Depictions of the ReStalsis Interferential Stimulator (RIS) device, Interferential Therapy (IFT) treatment method, and study timeline.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jenna Clukey indicated no relevant financial relationships.
Joseph Locascio indicated no relevant financial relationships.
Jack Semler: ATMO BioSciences – Independent Contractor. Restalsis – Independent Contractor.
Yogi Patel indicated no relevant financial relationships.
James Makous indicated no relevant financial relationships.
Ashok Attaluri: ReStalsis Health Inc. – Grant/Research Support.
Braden Kuo: restalsis – Consultant, Grant/Research Support.
Kyle Staller: Anji Pharmaceuticals – Consultant. Ardelyx – Consultant, Grant/Research Support. GI Supply – Consultant. Mahana Therapeutics – Consultant. Restalsis Health – Consultant, Grant/Research Support. Salix – Consultant.
Jenna Clukey, BA1, Joseph J.. Locascio, PhD2, Jack Semler, PhD3, Yogi Patel, PhD4, James Makous, PhD5, Ashok Attaluri, MD6, Braden Kuo, MD, MSc7, Kyle Staller, MD, MPH1. P2362 - A Multicenter, Sham-Controlled, Randomized Controlled Trial of Interferential Therapy for the Treatment of Chronic Constipation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


