Monday Poster Session
Category: IBD
P2543 - Ozanimod Improves Extraintestinal Manifestations in Patients With Ulcerative Colitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Wahibah Hannan, BS
Texas A&M School of Medicine
Houston, TX
Presenting Author(s)
Wahibah Hannan, BS1, Christopher Fan, MD2, Bincy P. Abraham, MD, MS, FACG3, Kerri Glassner, DO4
1Texas A&M School of Medicine, Houston, TX; 2Underwood Center for Digestive Disorders, Houston Methodist Hospital, Houston, TX; 3Houston Methodist-Weill Cornell, Houston, TX; 4Houston Methodist-Weill Cornell Graduate School of Medical Sciences, Houston, TX
Introduction: Ulcerative Colitis (UC) is a systemic disorder that can result in extraintestinal manifestations (EIM) that significantly impact quality of life. Ozanimod (OZD), a sphingosine 1-phosphate receptor modulator, was approved by the FDA in 2021 for the treatment of UC. Currently, there is no literature that reports its effect on EIM. The aim of our study was to determine whether OZD improves EIM.
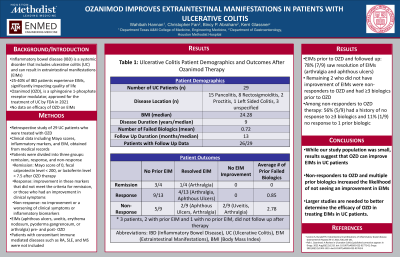
Methods: This was a retrospective study of 29 UC patients from a single institution who were treated with OZD. Clinical data including Mayo scores, inflammatory markers, and EIM, was obtained from medical records. The patients were divided into three groups based on their response to OZD: remission, response, and non-response. Remission was defined as having a Mayo score of 0, fecal calprotectin level < 200, or lactoferrin level < 7.5 after OZD therapy. The response group was defined as having an improvement in these markers that did not meet the criteria for remission, or those who had an improvement in clinical symptoms. The non-response group was defined as having no improvement or a worsening of clinical symptoms or inflammatory biomarkers. The presence of EIM (aphthous ulcers, uveitis, erythema nodosum, pyoderma gangrenosum, or arthralgia) before and after starting OZD was compared. Concomitant immune mediated diseases such as RA, SLE, and MS were not included.
Results: In the remission group (n=4), 3 patients reported no prior EIM and 1 reported a resolution of their EIM after OZD. In the response group (n=13), 9 reported no prior EIM and 4 reported a resolution of their EIM. For patients that were non-responders (n=9), 5 had no prior EIM, 2 reported a resolution of their EIM, and 2 reported no change in their EIM. Three patients, 2 with prior EIM, did not have follow up data available. For patients that had EIM prior to OZD and followed up, 78% (7/9) saw resolution of their EIM (arthralgia and aphthous ulcers). The 2 patients that did not see an improvement in their EIM were non-responders to OZD and had ≥3 biologics prior to OZD. Of the patients that did not respond to OZD therapy, 56% (5/9) had already had lack of response to ≥3 biologics and 11% (1/9) had not responded to 1 prior biologic.
Discussion: While our study population was small, our results suggest that OZD can improve EIM. Non-responders to OZD and multiple prior biologics increased the likelihood of not seeing an improvement in EIM. Larger studies are needed to better determine the efficacy of OZD in treating EIM in UC patients.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Wahibah Hannan, BS1, Christopher Fan, MD2, Bincy P. Abraham, MD, MS, FACG3, Kerri Glassner, DO4. P2543 - Ozanimod Improves Extraintestinal Manifestations in Patients With Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Texas A&M School of Medicine, Houston, TX; 2Underwood Center for Digestive Disorders, Houston Methodist Hospital, Houston, TX; 3Houston Methodist-Weill Cornell, Houston, TX; 4Houston Methodist-Weill Cornell Graduate School of Medical Sciences, Houston, TX
Introduction: Ulcerative Colitis (UC) is a systemic disorder that can result in extraintestinal manifestations (EIM) that significantly impact quality of life. Ozanimod (OZD), a sphingosine 1-phosphate receptor modulator, was approved by the FDA in 2021 for the treatment of UC. Currently, there is no literature that reports its effect on EIM. The aim of our study was to determine whether OZD improves EIM.
Methods: This was a retrospective study of 29 UC patients from a single institution who were treated with OZD. Clinical data including Mayo scores, inflammatory markers, and EIM, was obtained from medical records. The patients were divided into three groups based on their response to OZD: remission, response, and non-response. Remission was defined as having a Mayo score of 0, fecal calprotectin level < 200, or lactoferrin level < 7.5 after OZD therapy. The response group was defined as having an improvement in these markers that did not meet the criteria for remission, or those who had an improvement in clinical symptoms. The non-response group was defined as having no improvement or a worsening of clinical symptoms or inflammatory biomarkers. The presence of EIM (aphthous ulcers, uveitis, erythema nodosum, pyoderma gangrenosum, or arthralgia) before and after starting OZD was compared. Concomitant immune mediated diseases such as RA, SLE, and MS were not included.
Results: In the remission group (n=4), 3 patients reported no prior EIM and 1 reported a resolution of their EIM after OZD. In the response group (n=13), 9 reported no prior EIM and 4 reported a resolution of their EIM. For patients that were non-responders (n=9), 5 had no prior EIM, 2 reported a resolution of their EIM, and 2 reported no change in their EIM. Three patients, 2 with prior EIM, did not have follow up data available. For patients that had EIM prior to OZD and followed up, 78% (7/9) saw resolution of their EIM (arthralgia and aphthous ulcers). The 2 patients that did not see an improvement in their EIM were non-responders to OZD and had ≥3 biologics prior to OZD. Of the patients that did not respond to OZD therapy, 56% (5/9) had already had lack of response to ≥3 biologics and 11% (1/9) had not responded to 1 prior biologic.
Discussion: While our study population was small, our results suggest that OZD can improve EIM. Non-responders to OZD and multiple prior biologics increased the likelihood of not seeing an improvement in EIM. Larger studies are needed to better determine the efficacy of OZD in treating EIM in UC patients.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Wahibah Hannan indicated no relevant financial relationships.
Christopher Fan indicated no relevant financial relationships.
Bincy P. Abraham: AbbVie – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Janssen – Consultant, Speakers Bureau. Medtronic – Consultant. Pfizer – Consultant, Speakers Bureau. Prometheus – Consultant. Samsung Bioepis – Consultant. Takeda – Consultant, Speakers Bureau.
Kerri Glassner: Eli Lilly – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member. Pfizer – Advisor or Review Panel Member, Speakers Bureau.
Wahibah Hannan, BS1, Christopher Fan, MD2, Bincy P. Abraham, MD, MS, FACG3, Kerri Glassner, DO4. P2543 - Ozanimod Improves Extraintestinal Manifestations in Patients With Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

