Monday Poster Session
Category: IBD
P2544 - Model-Informed Dosing Interval Extension of Infliximab in Patients With Inflammatory Bowel Diseases: Six Months Interim Analysis of the MODIFI Trial
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Zhigang Wang, MSc, PharmD
Katholieke Universiteit Leuven
Leuven, Vlaams-Brabant, Belgium
Presenting Author(s)
Award: Presidential Poster Award
Zhigang Wang, MSc, PharmD1, Ine De Greef, MS, MD1, Wannee Kantasiripitak, MS, PhD1, Sebastian G. Wicha, PhD2, Sophie Tops, PhD1, Melissa Nigro, MS3, An Outtier, PhD3, Debby Thomas, PhD1, Séverine Vermeire, MD, PhD4, Marc Ferrante, MD, PhD3, Erwin Dreesen, PharmD; PhD1
1Katholieke Universiteit Leuven, Leuven, Vlaams-Brabant, Belgium; 2Universität Hamburg, Hamburg, Hamburg, Germany; 3University Hospitals, Leuven, Vlaams-Brabant, Belgium; 4University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium
Introduction: Extending the infliximab (IFX) infusion interval has been attempted in patients with Crohn’s disease (CD) and ulcerative colitis (UC) who sustained treatment response following an earlier interval shortening. We aimed to compare IFX interval extension based on therapeutic drug monitoring (TDM) with interval extension guided by a model-informed precision dosing (MIPD) software tool in patients with CD and UC.
Methods: MODIFI is a monocentric, open-label, historically controlled Phase 4 trial (NCT04982172). Eligible patients with steroid-free clinical and biological remission (two-item patient-reported outcome [PRO2] ≤1 for UC and ≤8 for CD, and C-reactive protein [CRP] < 5 mg/L and fecal calprotectin [FC] < 250 mg/kg) on less than 8-weekly IFX infusion schedules underwent interval extension to every eight weeks, guided by TDM (historical control) or MIPD targeting an IFX trough concentration (Cmin) of 5 mg/L with ≥80% probability of target attainment (prospective intervention). The primary endpoint of relapse-free survival was evaluated at each study visit over 52 weeks. IFX concentrations were measured right before every infusion. Bayesian forecasting was performed using the TDMx Infliximab module (www.TDMx.eu).
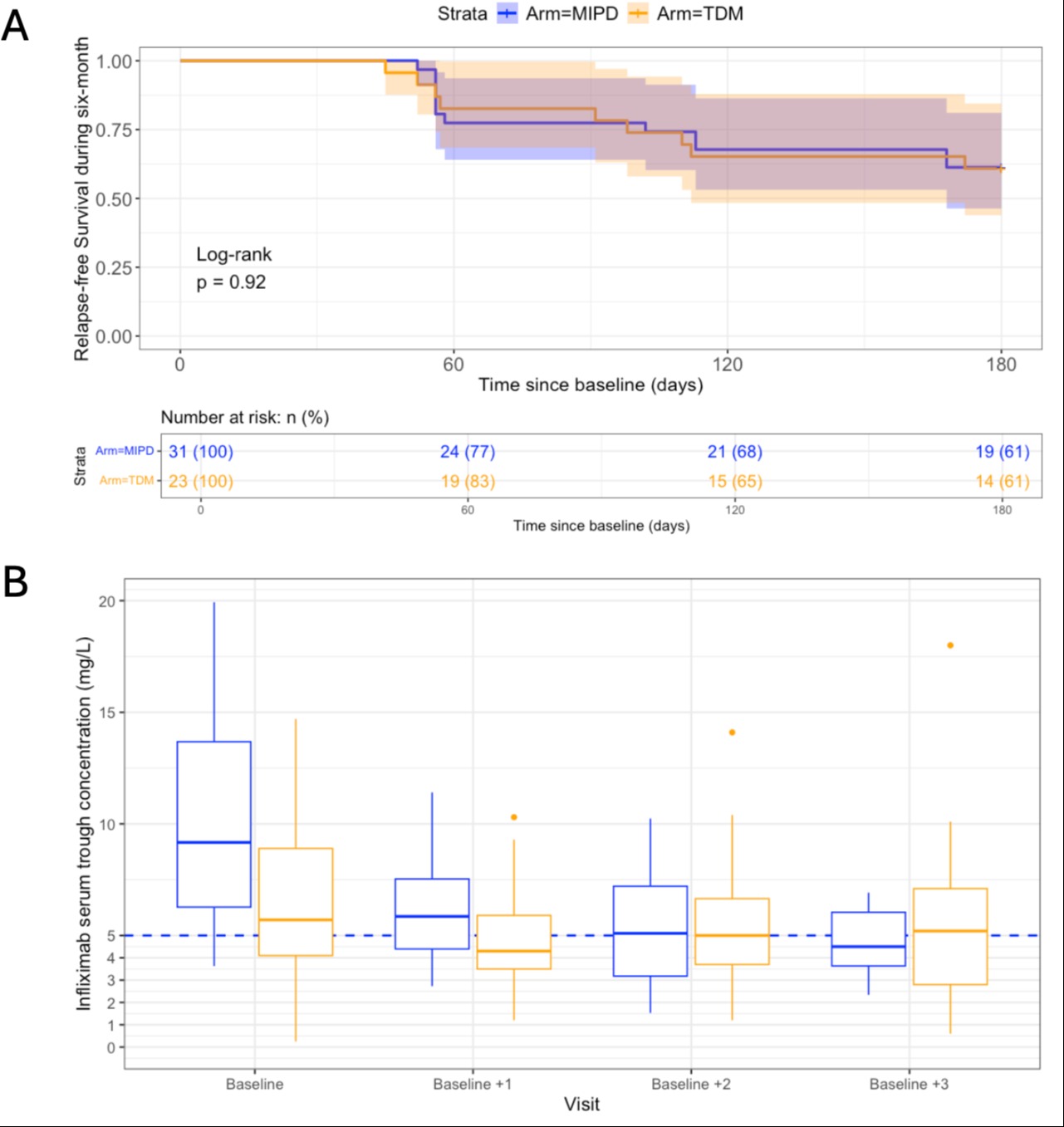
Results: The study enrolled 23 adult patients in the TDM arm (12 CD, 11 UC) and 31 in the MIPD arm (20 CD, 11 UC). In total, 61% (n=14/23) and 61% (n=19/31) of patients in the TDM and MIPD arms, respectively, remained relapse-free until six months after IFX interval extension (Figure 1A).
The median 8-weekly IFX dose per kg bodyweight at screening was significantly higher in the MIPD arm (11.7 [interquartile range IQR 6.8–14.9] mg/kg) than in the TDM arm (6.7 [IQR 6.1–8.4] mg/kg) (p < 0.050). The median IFX Cmin at baseline was significantly higher in the MIPD arm (9.2 [6.3–13.7] mg/L) than in the TDM arm (5.7 [4.2–9.1] mg/L) (p < 0.010). Following a single model-informed dose at baseline, the IFX Cmin immediately decreased to median 5.9 mg/L [IQR 4.4–7.5 mg/L] with 65% of patients >5 mg/L (Figure 1B). MIPD resulted a high classification accuracy of 83% at the Cmin target of 5 mg/L (the number of true positive and true negative predictions divided by the total number of predictions)
The median 8-weekly dose over six months was not significantly different between the MIPD and TDM arms (8.5 mg/kg [IQR 6.0–10.4] and 7.5 mg/kg [IQR 7.4–8.5], respectively).
Discussion: MIPD was equally effective as TDM to guide IFX interval extension in patients with CD and UC.
Disclosures:
Zhigang Wang, MSc, PharmD1, Ine De Greef, MS, MD1, Wannee Kantasiripitak, MS, PhD1, Sebastian G. Wicha, PhD2, Sophie Tops, PhD1, Melissa Nigro, MS3, An Outtier, PhD3, Debby Thomas, PhD1, Séverine Vermeire, MD, PhD4, Marc Ferrante, MD, PhD3, Erwin Dreesen, PharmD; PhD1. P2544 - Model-Informed Dosing Interval Extension of Infliximab in Patients With Inflammatory Bowel Diseases: Six Months Interim Analysis of the MODIFI Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Zhigang Wang, MSc, PharmD1, Ine De Greef, MS, MD1, Wannee Kantasiripitak, MS, PhD1, Sebastian G. Wicha, PhD2, Sophie Tops, PhD1, Melissa Nigro, MS3, An Outtier, PhD3, Debby Thomas, PhD1, Séverine Vermeire, MD, PhD4, Marc Ferrante, MD, PhD3, Erwin Dreesen, PharmD; PhD1
1Katholieke Universiteit Leuven, Leuven, Vlaams-Brabant, Belgium; 2Universität Hamburg, Hamburg, Hamburg, Germany; 3University Hospitals, Leuven, Vlaams-Brabant, Belgium; 4University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium
Introduction: Extending the infliximab (IFX) infusion interval has been attempted in patients with Crohn’s disease (CD) and ulcerative colitis (UC) who sustained treatment response following an earlier interval shortening. We aimed to compare IFX interval extension based on therapeutic drug monitoring (TDM) with interval extension guided by a model-informed precision dosing (MIPD) software tool in patients with CD and UC.
Methods: MODIFI is a monocentric, open-label, historically controlled Phase 4 trial (NCT04982172). Eligible patients with steroid-free clinical and biological remission (two-item patient-reported outcome [PRO2] ≤1 for UC and ≤8 for CD, and C-reactive protein [CRP] < 5 mg/L and fecal calprotectin [FC] < 250 mg/kg) on less than 8-weekly IFX infusion schedules underwent interval extension to every eight weeks, guided by TDM (historical control) or MIPD targeting an IFX trough concentration (Cmin) of 5 mg/L with ≥80% probability of target attainment (prospective intervention). The primary endpoint of relapse-free survival was evaluated at each study visit over 52 weeks. IFX concentrations were measured right before every infusion. Bayesian forecasting was performed using the TDMx Infliximab module (www.TDMx.eu).
Results: The study enrolled 23 adult patients in the TDM arm (12 CD, 11 UC) and 31 in the MIPD arm (20 CD, 11 UC). In total, 61% (n=14/23) and 61% (n=19/31) of patients in the TDM and MIPD arms, respectively, remained relapse-free until six months after IFX interval extension (Figure 1A).
The median 8-weekly IFX dose per kg bodyweight at screening was significantly higher in the MIPD arm (11.7 [interquartile range IQR 6.8–14.9] mg/kg) than in the TDM arm (6.7 [IQR 6.1–8.4] mg/kg) (p < 0.050). The median IFX Cmin at baseline was significantly higher in the MIPD arm (9.2 [6.3–13.7] mg/L) than in the TDM arm (5.7 [4.2–9.1] mg/L) (p < 0.010). Following a single model-informed dose at baseline, the IFX Cmin immediately decreased to median 5.9 mg/L [IQR 4.4–7.5 mg/L] with 65% of patients >5 mg/L (Figure 1B). MIPD resulted a high classification accuracy of 83% at the Cmin target of 5 mg/L (the number of true positive and true negative predictions divided by the total number of predictions)
The median 8-weekly dose over six months was not significantly different between the MIPD and TDM arms (8.5 mg/kg [IQR 6.0–10.4] and 7.5 mg/kg [IQR 7.4–8.5], respectively).
Discussion: MIPD was equally effective as TDM to guide IFX interval extension in patients with CD and UC.

Figure: Figure 1. (A) Relapse-free survival during six months after the start of infliximab therapy. (B) Infliximab serum trough concentrations at every study visit in the model-informed precision dosing (MIPD, colored in blue) and therapeutic drug monitoring (TDM, colored in orange) arm. MIPD was started from the Baseline onwards in the MIPD arm.
Disclosures:
Zhigang Wang: Celltrion – Sponsorship of travel and accomendation cost for ACG2024 conference.
Ine De Greef indicated no relevant financial relationships.
Wannee Kantasiripitak indicated no relevant financial relationships.
Sebastian G. Wicha indicated no relevant financial relationships.
Sophie Tops indicated no relevant financial relationships.
Melissa Nigro indicated no relevant financial relationships.
An Outtier indicated no relevant financial relationships.
Debby Thomas indicated no relevant financial relationships.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. Abivax – Consultant. AbolerlsPharma – Consultant. AgomAb – Consultant. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. BioraTherapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Cytoki Pharma – Consultant. Dr Falk Pharma – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Galapagos – Consultant, Grant/Research Support. Genentech Roche – Consultant. Gilead – Consultant. GSK – Consultant. Hospira – Consultant. J&J – Consultant, Grant/Research Support. Janssen – Consultant. lmidomics – Consultant. Materia Prima – Consultant. Mestag Therapeutics – Consultant. Microbiotica – Consultant. MiroBio – Consultant. Morphic – Consultant. MrMHealth – Consultant. MSD – Consultant. Mundipharma – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prodigest – Consultant. Progenity – Consultant. Prometheus – Consultant. Robarts Clinical Trials – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. Theravance – Consultant. Tillots Pharma AG – Consultant. VectivBio – Consultant. Ventyx – Consultant. Zealand Pharma – Consultant.
Marc Ferrante: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Agomab – Consultant. Amgen – Grant/Research Support, Speakers Bureau. Biogen – Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant. Celltrion – Consultant. Dr Falk Pharma – Speakers Bureau. EG Pharmaceuticals – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support. Ferring – Speakers Bureau. Janssen – Grant/Research Support. Janssen-Cilag – Consultant, Speakers Bureau. Lamepro – Speakers Bureau. Medtronic – Consultant. MRM Health – Consultant. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Regeneron – Consultant. Samsung Bioepis – Consultant. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. ThermoFisher – Consultant. Truvion Healthcare – Speakers Bureau. Viatris – Grant/Research Support, Speakers Bureau.
Erwin Dreesen: Alimentiv – Consultant. argenx – Consultant. Celltrion – Speakers Bureau. Galapagos – Speakers Bureau. Janssen – Grant/Research Support. Prometheus – Grant/Research Support. Sandoz – Grant/Research Support.
Zhigang Wang, MSc, PharmD1, Ine De Greef, MS, MD1, Wannee Kantasiripitak, MS, PhD1, Sebastian G. Wicha, PhD2, Sophie Tops, PhD1, Melissa Nigro, MS3, An Outtier, PhD3, Debby Thomas, PhD1, Séverine Vermeire, MD, PhD4, Marc Ferrante, MD, PhD3, Erwin Dreesen, PharmD; PhD1. P2544 - Model-Informed Dosing Interval Extension of Infliximab in Patients With Inflammatory Bowel Diseases: Six Months Interim Analysis of the MODIFI Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

