Monday Poster Session
Category: IBD
P2596 - Clinical Effectiveness of Vedolizumab in Chinese Patients for Ulcerative Colitis: Interim Results From the VALUE study
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- YC
Yan Chen
The Second Affiliated Hospital, Zhejiang University School of Medicine
Hangzhou, Zhejiang, China
Presenting Author(s)
Yan Chen, 1, Yihong Fan, 2, Xiaoyu Qian, 3, Li Xie, 4, Minhu Chen, MD, PhD5
1The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China; 2Zhejiang Provincial Hospital of Chinese Medicine, Hangzhou, Zhejiang, China; 3Takeda Pharmaceutical Company, Shanghai, Shanghai, China; 4Takeda Pharmaceutical Company Ltd., Shanghai, Shanghai, China; 5The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China
Introduction: Vedolizumab (VDZ), an advanced therapy with unique gut-selective anti-lymphocyte trafficking mechanism was approved to treat moderate-to-severe Ulcerative Colitis (UC) and Crohn’s disease (CD) patients (pts) in 2020 in China. However, the effectiveness and safety data of VDZ in Chinese Inflammatory bowel disease (IBD) pts is lacking.
Methods: VALUE study is a prospective, multicenter, single-armed observational study and included 500 IBD adult pts (NCT04872491). Effectiveness of VDZ was evaluated for a 54-week observation period and included clinical response, clinical remission, and endoscopic remission at weeks 14 (W14), 30 (W30), and 54 (W54). Partial Mayo and Mayo endoscopic sub-scores at baseline and W14, W30, W54 (mean ± standard deviation [SD]) were calculated. This is the second interim analysis of VALUE study in UC pts. Safety results are presented in another VALUE abstract (ID:1859655)
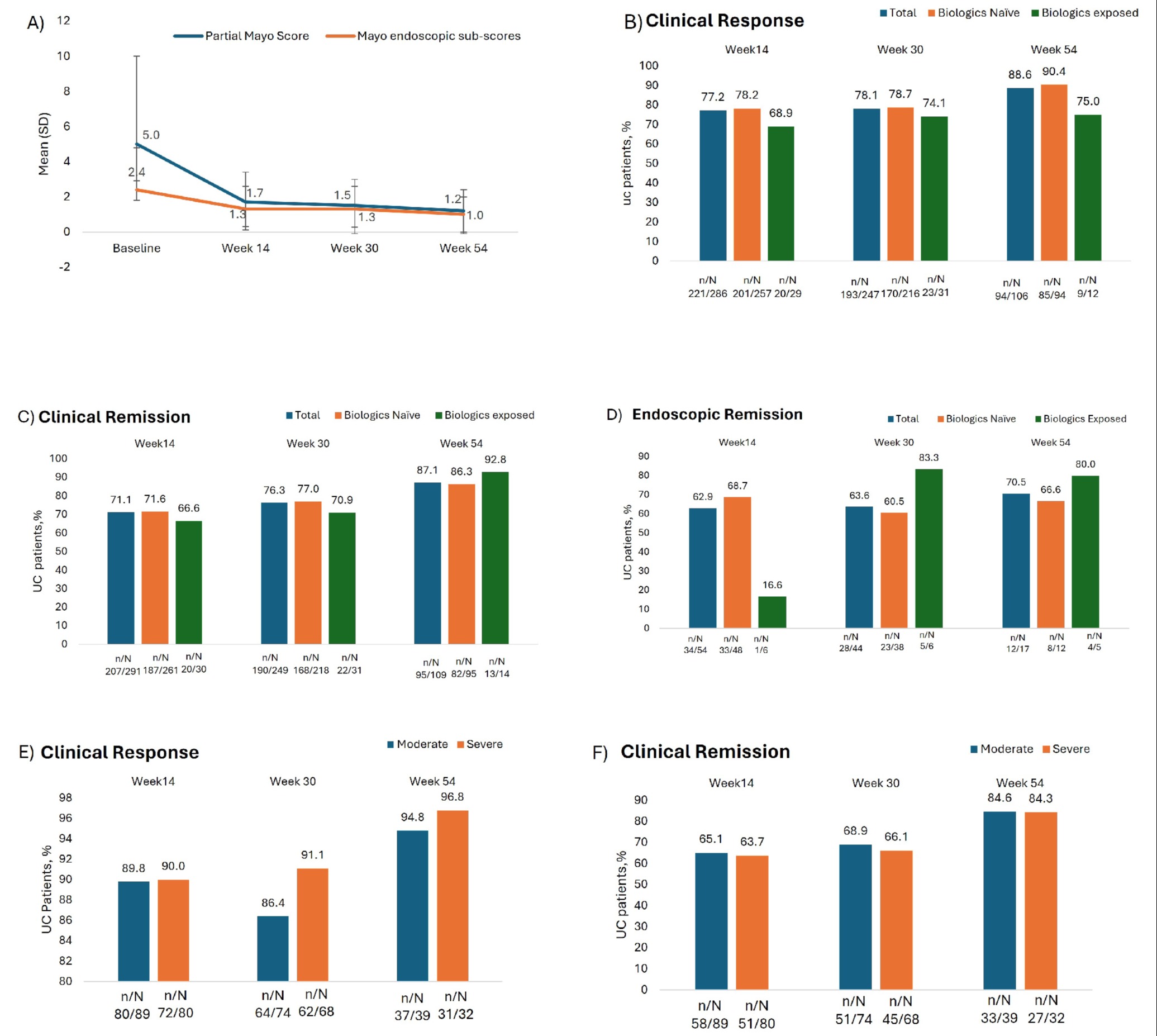
Results: Of the 409 UC pts enrolled, 91.6% (375/409) were included in the effectiveness analysis set. Mean age was 46.3 years and 59.6% (244/409) pts were male. Most pts were with extensive UC (53.0%), median duration of UC was 2.50year and 10.5% (43/409) pts had prior biologic use with Partial Mayo mean (±SD) score of 5.2 (±2.09) (Table 1). Compared with baseline, both partial Mayo score and Mayo endoscopic scores decreased at weeks 14, 30 and 54 (Fig.1A). As observed, the proportion of pts achieving clinical response at W14, W30, and W54 were 77.2% (221/286), 78.1% (193/247), and 88.6% (94/106), respectively. Proportion of pts achieving clinical remission at W14, W30, and W54 were: 71.1% (207/291), 76.3% (190/249), and 87.1% (95/109) respectively. Endoscopic remission (defined as Mayo endoscopic sub-score ≤1) and complete endoscopic remission (defined as Mayo endoscopic sub-score =0) were 62.9% (34/54) and 22.2% (12/54) at W14, 63.6 % (28/44) and 27.2% (12/44) at W30, 70.5% (12/17) and 41.1% (7/17) at W54. Results of clinical response, clinical remission, and endoscopic remission at weeks 14, 30, and 54 in subgroups stratified by bio-exposure are shown in Fig1B-D, those in subgroup of moderate UC and severe UC are shown in Fig 1E-G. The 59 (15.73%) pts reported concomitant IBD steroid use at baseline were included in effectiveness analysis. The proportion of pts achieving clinical remission without steroid use at W14 was 38.46% (20/42) and at W54 was 65% (13/18).
Discussion: VDZ treatment was effective and well tolerated in Chinese UC pts in the real-world setting.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Yan Chen, 1, Yihong Fan, 2, Xiaoyu Qian, 3, Li Xie, 4, Minhu Chen, MD, PhD5. P2596 - Clinical Effectiveness of Vedolizumab in Chinese Patients for Ulcerative Colitis: Interim Results From the VALUE study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China; 2Zhejiang Provincial Hospital of Chinese Medicine, Hangzhou, Zhejiang, China; 3Takeda Pharmaceutical Company, Shanghai, Shanghai, China; 4Takeda Pharmaceutical Company Ltd., Shanghai, Shanghai, China; 5The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China
Introduction: Vedolizumab (VDZ), an advanced therapy with unique gut-selective anti-lymphocyte trafficking mechanism was approved to treat moderate-to-severe Ulcerative Colitis (UC) and Crohn’s disease (CD) patients (pts) in 2020 in China. However, the effectiveness and safety data of VDZ in Chinese Inflammatory bowel disease (IBD) pts is lacking.
Methods: VALUE study is a prospective, multicenter, single-armed observational study and included 500 IBD adult pts (NCT04872491). Effectiveness of VDZ was evaluated for a 54-week observation period and included clinical response, clinical remission, and endoscopic remission at weeks 14 (W14), 30 (W30), and 54 (W54). Partial Mayo and Mayo endoscopic sub-scores at baseline and W14, W30, W54 (mean ± standard deviation [SD]) were calculated. This is the second interim analysis of VALUE study in UC pts. Safety results are presented in another VALUE abstract (ID:1859655)
Results: Of the 409 UC pts enrolled, 91.6% (375/409) were included in the effectiveness analysis set. Mean age was 46.3 years and 59.6% (244/409) pts were male. Most pts were with extensive UC (53.0%), median duration of UC was 2.50year and 10.5% (43/409) pts had prior biologic use with Partial Mayo mean (±SD) score of 5.2 (±2.09) (Table 1). Compared with baseline, both partial Mayo score and Mayo endoscopic scores decreased at weeks 14, 30 and 54 (Fig.1A). As observed, the proportion of pts achieving clinical response at W14, W30, and W54 were 77.2% (221/286), 78.1% (193/247), and 88.6% (94/106), respectively. Proportion of pts achieving clinical remission at W14, W30, and W54 were: 71.1% (207/291), 76.3% (190/249), and 87.1% (95/109) respectively. Endoscopic remission (defined as Mayo endoscopic sub-score ≤1) and complete endoscopic remission (defined as Mayo endoscopic sub-score =0) were 62.9% (34/54) and 22.2% (12/54) at W14, 63.6 % (28/44) and 27.2% (12/44) at W30, 70.5% (12/17) and 41.1% (7/17) at W54. Results of clinical response, clinical remission, and endoscopic remission at weeks 14, 30, and 54 in subgroups stratified by bio-exposure are shown in Fig1B-D, those in subgroup of moderate UC and severe UC are shown in Fig 1E-G. The 59 (15.73%) pts reported concomitant IBD steroid use at baseline were included in effectiveness analysis. The proportion of pts achieving clinical remission without steroid use at W14 was 38.46% (20/42) and at W54 was 65% (13/18).
Discussion: VDZ treatment was effective and well tolerated in Chinese UC pts in the real-world setting.

Figure: Figure 1: A) Mean Partial Mayo and Mayo endoscopic sub-scores at baseline, week14, week30, and week54; B) Clinical Response, C) Clinical Remission, and D) Endoscopic Remission rates at weeks 14, 30, and 54 in subgroups of bio-exposure; E) Clinical Response, F) Clinical Remission and F) Endoscopic remission rates at weeks 14, 30 and 54 in subgroups of moderate UC and severe UC.
aClinical response defined as ≥2 points reduction in Partial Mayo Clinic score and ≥25% decrease from baseline score accompanied with ≥1-point decrease in rectal bleeding sub-score or absolute rectal bleeding sub-score ≤1; bClinical remission defined as Partial Mayo Clinic score ≤2 with no sub-score >1; cEndoscopic remission defined as Mayo endoscopic sub-score ≤1.
aClinical response defined as ≥2 points reduction in Partial Mayo Clinic score and ≥25% decrease from baseline score accompanied with ≥1-point decrease in rectal bleeding sub-score or absolute rectal bleeding sub-score ≤1; bClinical remission defined as Partial Mayo Clinic score ≤2 with no sub-score >1; cEndoscopic remission defined as Mayo endoscopic sub-score ≤1.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Yan Chen indicated no relevant financial relationships.
Yihong Fan indicated no relevant financial relationships.
Xiaoyu Qian indicated no relevant financial relationships.
Li Xie: Takeda China – Employee, Stock Options.
Minhu Chen indicated no relevant financial relationships.
Yan Chen, 1, Yihong Fan, 2, Xiaoyu Qian, 3, Li Xie, 4, Minhu Chen, MD, PhD5. P2596 - Clinical Effectiveness of Vedolizumab in Chinese Patients for Ulcerative Colitis: Interim Results From the VALUE study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
